Improved transduction of canine X-linked muscular dystrophy with rAAV9-microdystrophin via multipotent MSC pretreatment
- PMID: 33426145
- PMCID: PMC7773564
- DOI: 10.1016/j.omtm.2020.11.003
Improved transduction of canine X-linked muscular dystrophy with rAAV9-microdystrophin via multipotent MSC pretreatment
Abstract
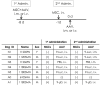
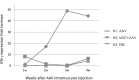
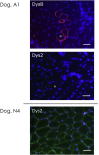
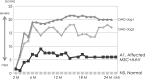
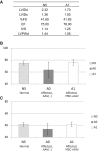
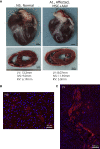
Duchenne muscular dystrophy (DMD) is a severe congenital disease associated with mutation of the dystrophin gene. Supplementation of dystrophin using recombinant adeno-associated virus (rAAV) has promise as a treatment for DMD, although vector-related general toxicities, such as liver injury, neurotoxicity, and germline transmission, have been suggested in association with the systemic delivery of high doses of rAAV. Here, we treated normal or dystrophic dogs with rAAV9 transduction in conjunction with multipotent mesenchymal stromal cell (MSC) injection to investigate the therapeutic effects of an rAAV expressing microdystrophin (μDys) under conditions of immune modulation. Bone-marrow-derived MSCs, rAAV-CMV-μDys, and a rAAV-CAG-luciferase (Luc) were injected into the jugular vein of a young dystrophic dog to induce systemic expression of μDys. One week after the first injection, the dog received a second intravenous injection of MSCs, and on the following day, rAAV was intravenously injected into the same dog. Systemic injection of rAAV9 with MSCs pretreatment improves gene transfer into normal and dystrophic dogs. Dystrophic phenotypes significantly improved in the rAAV-μDys-injected dystrophic dog, suggesting that an improved rAAV-μDys treatment including immune modulation induces successful long-term transgene expression to improve dystrophic phenotypes.
Keywords: AAV; DMD; DMD dog model; MSC; immune modulation; microdystrophin.
© 2020 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Intra-amniotic rAAV-mediated microdystrophin gene transfer improves canine X-linked muscular dystrophy and may induce immune tolerance.Mol Ther. 2015 Apr;23(4):627-37. doi: 10.1038/mt.2015.5. Epub 2015 Jan 14. Mol Ther. 2015. PMID: 25586688 Free PMC article.
-
Long-term functional adeno-associated virus-microdystrophin expression in the dystrophic CXMDj dog.J Gene Med. 2011 Sep;13(9):497-506. doi: 10.1002/jgm.1602. J Gene Med. 2011. PMID: 22144143
-
Long-term microdystrophin gene therapy is effective in a canine model of Duchenne muscular dystrophy.Nat Commun. 2017 Jul 25;8:16105. doi: 10.1038/ncomms16105. Nat Commun. 2017. PMID: 28742067 Free PMC article.
-
[Current status and perspective of gene therapy on dystrophic animal model].Rinsho Shinkeigaku. 2004 Nov;44(11):911-3. Rinsho Shinkeigaku. 2004. PMID: 15651329 Review. Japanese.
-
Recombinant adeno-associated viral (rAAV) vectors as therapeutic tools for Duchenne muscular dystrophy (DMD).Gene Ther. 2004 Oct;11 Suppl 1:S109-21. doi: 10.1038/sj.gt.3302379. Gene Ther. 2004. PMID: 15454965 Review.
Cited by
-
Protected Geographical Indication Discrimination of Zhejiang and Non-Zhejiang Ophiopogonis japonicus by Near-Infrared (NIR) Spectroscopy Combined with Chemometrics: The Influence of Different Stoichiometric and Spectrogram Pretreatment Methods.Molecules. 2023 Mar 20;28(6):2803. doi: 10.3390/molecules28062803. Molecules. 2023. PMID: 36985775 Free PMC article.
-
AAV microdystrophin gene replacement therapy for Duchenne muscular dystrophy: progress and prospects.Gene Ther. 2025 Aug 15. doi: 10.1038/s41434-025-00561-6. Online ahead of print. Gene Ther. 2025. PMID: 40817386 Review.
-
Development of outcome measures according to dystrophic phenotypes in canine X-linked muscular dystrophy in Japan.Exp Anim. 2021 Nov 10;70(4):419-430. doi: 10.1538/expanim.21-0072. Epub 2021 Jun 16. Exp Anim. 2021. PMID: 34135266 Free PMC article.
-
Therapeutic approaches for Duchenne muscular dystrophy.Nat Rev Drug Discov. 2023 Nov;22(11):917-934. doi: 10.1038/s41573-023-00775-6. Epub 2023 Aug 31. Nat Rev Drug Discov. 2023. PMID: 37652974 Review.
-
Stem Cell-Based Therapeutic Approaches in Genetic Diseases.Adv Exp Med Biol. 2023;1436:19-53. doi: 10.1007/5584_2023_761. Adv Exp Med Biol. 2023. PMID: 36735185
References
-
- Mendell J.R., Al-Zaidy S., Shell R., Arnold W.D., Rodino-Klapac L.R., Prior T.W., Lowes L., Alfano L., Berry K., Church K. Single-Dose Gene-Replacement Therapy for Spinal Muscular Atrophy. N. Engl. J. Med. 2017;377:1713–1722. - PubMed
-
- Arruda V.R., Fields P.A., Milner R., Wainwright L., De Miguel M.P., Donovan P.J., Herzog R.W., Nichols T.C., Biegel J.A., Razavi M. Lack of germline transmission of vector sequences following systemic administration of recombinant AAV-2 vector in males. Mol. Ther. 2001;4:586–592. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

