Recombinant canine basic fibroblast growth factor-induced differentiation of canine bone marrow mesenchymal stem cells into voltage- and glutamate-responsive neuron-like cells
- PMID: 33426210
- PMCID: PMC7770349
- DOI: 10.1016/j.reth.2020.07.005
Recombinant canine basic fibroblast growth factor-induced differentiation of canine bone marrow mesenchymal stem cells into voltage- and glutamate-responsive neuron-like cells
Abstract
Introduction: Basic fibroblast growth factor (bFGF) is a promising cytokine in regenerative therapy for spinal cord injury. In this study, recombinant canine bFGF (rc-bFGF) was synthesized for clinical use in dogs, and the ability of rc-bFGF to differentiate canine bone marrow mesenchymal stem cells (BMSCs) into functional neurons was investigated.
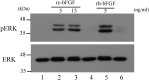
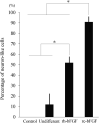
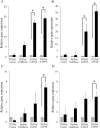
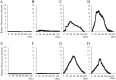
Methods: The rc-bFGF was synthesized using a wheat germ cell-free protein synthesis system. The expression of rc-bFGF mRNA in the purification process was confirmed using a reverse transcription-polymerase chain reaction (RT-PCR). Western blotting was performed to confirm the antigenic property of the purified protein. To verify function of the purified protein, phosphorylation of extracellular signal-regulated kinase (ERK) was examined by in vitro assay using HEK293 cells. To compare the neuronal differentiation capacity of canine BMSCs in response to treatment with rc-bFGF, the cells were divided into the following four groups: control, undifferentiated, rh-bFGF, and rc-bFGF groups. After neuronal induction, the percentage of cells that had changed to a neuron-like morphology and the mRNA expression of neuronal markers were evaluated. Furthermore, to assess the function of the canine BMSCs after neuronal induction, changes in the intracellular Ca2+ concentrations after stimulation with KCl and l-glutamate were examined.
Results: The protein synthesized in this study was rc-bFGF and functioned as bFGF, from the results of RT-PCR, western blotting, and the expression of pERK in HEK293 cells. Canine BMSCs acquired a neuron-like morphology and expressed mRNAs of neuronal markers after neuronal induction in the rh-bFGF and the rc-bFGF groups. These results were more marked in the rc-bFGF group than in the other groups. Furthermore, an increase in intracellular Ca2+ concentrations was observed after the stimulation of KCl and l-glutamate in the rc-bFGF group, same as in the rh-bFGF group.
Conclusions: A functional rc-bFGF was successfully synthesized, and rc-bFGF induced the differentiation of canine BMSCs into voltage- and glutamate-responsive neuron-like cells. Our purified rc-bFGF may contribute, on its own, or in combination with canine BMSCs, to regenerative therapy for spinal cord injury in dogs.
Keywords: BMSCs, bone marrow mesenchymal stem cells; Basic fibroblast growth factor; Bone marrow; Differentiation; Dog; EDTA, ethylenediaminetetraacetic acid; ERK, extracellular signal-regulated kinase; FBS, fatal bovine serum; FGFR, basic fibroblast growth factor receptor; GUSB, β-glucuronidase; HEK293, human embryonic kidney cells 293; HRP, horseradish peroxidase; Mesenchymal stem cell; Neuron; PBS, phosphate buffered saline; PCR, polymerase chain reaction; PI3K, phosphatidylinositol 3-kinase; RT-PCR, reverse transcription-polymerase chain reaction; bFGF, basic fibroblast growth factor; cDNA, complementary DNA; mRNA, messenger ribonucleic acid; pERK, phosphorylated extracellular signal-regulated kinase; αMEM, alpha modified eagle minimum essential medium.
© 2020 The Japanese Society for Regenerative Medicine. Production and hosting by Elsevier B.V.
Conflict of interest statement
None.
Figures



Similar articles
-
Differentiation of canine bone marrow stromal cells into voltage- and glutamate-responsive neuron-like cells by basic fibroblast growth factor.J Vet Med Sci. 2015 Jan;77(1):27-35. doi: 10.1292/jvms.14-0284. Epub 2014 Oct 6. J Vet Med Sci. 2015. PMID: 25284120 Free PMC article.
-
Fibroblast Growth Factor Receptor-2 Contributes to the Basic Fibroblast Growth Factor-Induced Neuronal Differentiation in Canine Bone Marrow Stromal Cells via Phosphoinositide 3-Kinase/Akt Signaling Pathway.PLoS One. 2015 Nov 2;10(11):e0141581. doi: 10.1371/journal.pone.0141581. eCollection 2015. PLoS One. 2015. PMID: 26523832 Free PMC article.
-
[Effects of three inducing factors on differentiation of bone marrow derived mesenchymal stem cells into lymphatic endothelial cells].Zhonghua Shao Shang Za Zhi. 2019 Feb 20;35(2):125-133. doi: 10.3760/cma.j.issn.1009-2587.2019.02.008. Zhonghua Shao Shang Za Zhi. 2019. PMID: 30798579 Chinese.
-
Evaluation of mRNA expression levels and electrophysiological function of neuron-like cells derived from canine bone marrow stromal cells.Am J Vet Res. 2013 Oct;74(10):1311-20. doi: 10.2460/ajvr.74.10.1311. Am J Vet Res. 2013. PMID: 24066915
-
[Effect of Basic Fibroblast Growth Factor and Transforming Growth Factor-Β1 Combined with Bone Marrow Mesenchymal Stem Cells on the Repair of Degenerated Intervertebral Discs in Rat Models].Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2015 Aug;37(4):456-65. doi: 10.3881/j.issn.1000-503X.2015.04.016. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2015. PMID: 26564465 Chinese.
Cited by
-
Growth Factors VEGF-A165 and FGF-2 as Multifunctional Biomolecules Governing Cell Adhesion and Proliferation.Int J Mol Sci. 2021 Feb 12;22(4):1843. doi: 10.3390/ijms22041843. Int J Mol Sci. 2021. PMID: 33673317 Free PMC article.
-
New Diagnostic and Therapeutic Targets for Spinal Cord Injury: GRN Gene.J Cell Mol Med. 2025 Aug;29(15):e70749. doi: 10.1111/jcmm.70749. J Cell Mol Med. 2025. PMID: 40736401 Free PMC article.
-
Milestones and current achievements in development of multifunctional bioscaffolds for medical application.Bioact Mater. 2021 Jan 28;6(8):2412-2438. doi: 10.1016/j.bioactmat.2021.01.007. eCollection 2021 Aug. Bioact Mater. 2021. PMID: 33553825 Free PMC article. Review.
-
MSC based gene delivery methods and strategies improve the therapeutic efficacy of neurological diseases.Bioact Mater. 2022 Nov 30;23:409-437. doi: 10.1016/j.bioactmat.2022.11.007. eCollection 2023 May. Bioact Mater. 2022. PMID: 36474656 Free PMC article. Review.
-
Molecular approaches for spinal cord injury treatment.Neural Regen Res. 2023 Jan;18(1):23-30. doi: 10.4103/1673-5374.344830. Neural Regen Res. 2023. PMID: 35799504 Free PMC article. Review.
References
-
- Prockop D.J. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. - PubMed
-
- Pittenger M.F., Mackay A.M., Beck S.C., Jaiswal R.K., Douglas R., Mosca J.D. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. - PubMed
-
- Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach, Marini F., Krause D. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cryotherapy. 2006;8:315–317. - PubMed
-
- Woodbury D., Schwarz E.J., Prockop D.J., Black I.B. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000;61:364–370. - PubMed
-
- Sanchez-Ramos J., Song S., Cardozo-Pelaez F., Hazzi C., Stedeford T. Adult bone marrow stromal cells differentiate into neural cells in vitro. Exp Neurol. 2000;164:247–256. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

