Urinary bladder reconstruction using autologous collagenous connective tissue membrane "Biosheet®" induced by in-body tissue architecture: A pilot study
- PMID: 33426229
- PMCID: PMC7770416
- DOI: 10.1016/j.reth.2020.10.006
Urinary bladder reconstruction using autologous collagenous connective tissue membrane "Biosheet®" induced by in-body tissue architecture: A pilot study
Abstract
Introduction: In-body tissue architecture (iBTA) technology, based on cell-free tissue engineering, can produces collagenous tissues for implantation by subcutaneous embedding a designed mold. The aim of this study was to evaluate the biocompatibility of iBTA-induced "Biosheet®" collagenous sheets, as scaffold materials for bladder reconstruction.
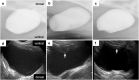
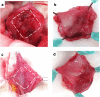
Methods: Canine Biosheet® implants were prepared by embedding molds into subcutaneous pouches in beagles for 8 weeks. A part of canine bladder wall was excised (2 × 2 cm) and repaired by patching the same sized autologous Biosheet®. The Biosheet® implants were harvested 4 weeks (n = 1) and 12 weeks (n = 3) after the implantation and evaluated histologically.
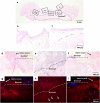
Results: No disruption of the patched Biosheet® implants or urinary leakage into the peritoneal cavity was observed during the entire observation periods. There were no signs of chronic inflammation or Biosheet® rejection. The urine-contacting surface of luminal surface of the Biosheet® was covered with a multicellular layer of urothelium cells 4 weeks after implantation. α-SMA-positive muscle cells were observed at the margin of the Biosheet® implants at 12 weeks after the implantation. In addition, in the center of the Biosheet® implants, the formation of microvessels stained as α-SMA-positive was observed.
Conclusion: Biosheet® implants have biocompatibility as a scaffold for bladder reconstruction, indicating that they may be applicable for full-thickness bladder wall substitution. Further studies are required for definitive evaluation as a scaffold for bladder reconstruction.
Keywords: BAM, bladder acellular matrices; Biosheet®; Bladder reconstruction; In body tissue architecture; Regenerative medicine; SIS, small intestinal submucosa; Tissue engineering; Urinary bladder; iBTA, in-body tissue architecture.
© 2020 The Japanese Society for Regenerative Medicine. Production and hosting by Elsevier B.V.
Conflict of interest statement
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Y.N. is an employee of Biotube Co., Ltd. The other authors declare no conflicts of interest associated with this manuscript.
Figures


Similar articles
-
Bladder Reconstruction in Cats Using In-Body Tissue Architecture (iBTA)-Induced Biosheet.Bioengineering (Basel). 2024 Jun 16;11(6):615. doi: 10.3390/bioengineering11060615. Bioengineering (Basel). 2024. PMID: 38927851 Free PMC article.
-
iBTA-induced bovine Biosheet for repair of abdominal wall defects in a beagle model: proof of concept.Hernia. 2018 Dec;22(6):1033-1039. doi: 10.1007/s10029-018-1799-8. Epub 2018 Jul 18. Hernia. 2018. PMID: 30022282
-
One-year follow-up study of iBTA-induced allogenic biosheet for repair of abdominal wall defects in a beagle model: a pilot study.Hernia. 2019 Feb;23(1):149-155. doi: 10.1007/s10029-018-1866-1. Epub 2018 Nov 30. Hernia. 2019. PMID: 30506241
-
Understanding roles of porcine small intestinal submucosa in urinary bladder regeneration: identification of variable regenerative characteristics of small intestinal submucosa.Tissue Eng Part B Rev. 2014 Feb;20(1):73-83. doi: 10.1089/ten.TEB.2013.0126. Epub 2013 Jul 25. Tissue Eng Part B Rev. 2014. PMID: 23777420 Free PMC article. Review.
-
Tissue engineering technologies: just a quick note about transplantation of bioengineered donor trachea and augmentation cystoplasty by de novo engineered bladder tissue.G Chir. 2009 Nov-Dec;30(11-12):514-9. G Chir. 2009. PMID: 20109384 Review.
Cited by
-
Successful reconstruction of the rat ureter by a syngeneic collagen tube with a cardiomyocyte sheet.Regen Ther. 2023 Oct 13;24:561-567. doi: 10.1016/j.reth.2023.10.001. eCollection 2023 Dec. Regen Ther. 2023. PMID: 37868722 Free PMC article.
-
Recent advances in innovative biomaterials for promoting bladder regeneration: processing and functionalization.Front Bioeng Biotechnol. 2025 Jan 6;12:1528658. doi: 10.3389/fbioe.2024.1528658. eCollection 2024. Front Bioeng Biotechnol. 2025. PMID: 39834643 Free PMC article. Review.
-
Strategies of Bladder Reconstruction after Partial or Radical Cystectomy for Bladder Cancer.Mol Biotechnol. 2025 May;67(5):1735-1751. doi: 10.1007/s12033-024-01163-0. Epub 2024 May 18. Mol Biotechnol. 2025. PMID: 38761327 Review.
-
Research progress of biomaterials and innovative technologies in urinary tissue engineering.Front Bioeng Biotechnol. 2023 Aug 14;11:1258666. doi: 10.3389/fbioe.2023.1258666. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 37645598 Free PMC article. Review.
-
Augmentation cystoplasty in dogs: A comparative study of different tunica vaginalis grafts.Vet Anim Sci. 2022 Mar 18;16:100247. doi: 10.1016/j.vas.2022.100247. eCollection 2022 Jun. Vet Anim Sci. 2022. PMID: 35345763 Free PMC article.
References
-
- Mingin G.C., Stock J.A., Hanna M.K. Gastrocystoplasty: long-term complications in 22 patients. J Urol. 1999;162:1122–1125. - PubMed
-
- Kurzrock E.A. Pediatric enterocystoplasty: long-term complications and controversies. World J Urol. 2009;27:69–73. - PubMed
-
- Rousson B., Verzeaux E., Leriche A. Urethroplasty using polyglactin mesh in urethral fistula caused by decubitus ulcer of the perineum in spinal cord injuries. Apropos of 7 cases. Ann Chir Plast Esthet. 1994;39:10–14. - PubMed
-
- Baltaci S., Ozer G., Ozer E., Soygür T., Beşalti O., Anafarta K. Failure of ureteral replacement with Gore-Tex tube grafts. Urology. 1998;51:400–403. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

