Stem cell therapy for osteonecrosis of femoral head: Opportunities and challenges
- PMID: 33426232
- PMCID: PMC7770428
- DOI: 10.1016/j.reth.2020.11.003
Stem cell therapy for osteonecrosis of femoral head: Opportunities and challenges
Abstract
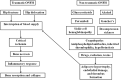
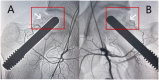
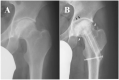
Osteonecrosis of the femoral head (ONFH) is a progressive disease with a complex etiology and unclear pathogenesis, resulting in severe hip pain and dysfunction mainly observed in young patients. Although total hip arthroplasty (THA) is the most effective treatment for patients with ONFH in the terminal stage, the results of THA in young patients or active populations are often not favorable, with some complications related to the prosthesis. With the development of biotechnology, an increasing number of studies pay attention to use of stem cells for the treatment of ONFH. Stem cells are characterized by the ability to self-renew and differentiate into multiple cell types, including differentiation into osteoblasts and endothelial cells to mediate bone repair and angiogenesis. Furthermore, stem cells can offer growth factors to promote blood supply in the necrotic regions by paracrine effects. Therefore, stem cell therapy has become one of the hip-preserving alternatives for ONFH. This review summarized the current trends in stem cell therapy for ONFH, from clinical applications to related basic research, and showed that an increasing number of studies have confirmed the effectiveness of stem cell therapy in ONFH. However, many unsolved problems and challenges in practical applications of stem cell therapy still exist, such as patient selection, standardized procedures, safety assessment, and the fate of transplanted cells in the body. Additional studies are required to find ideal cell sources, appropriate transplantation methods, and the optimal number of cells for transplantation.
Keywords: ALP, alkaline phosphatase; AMSCs, adipose-derived MSCs; BCP, biphasic calcium phosphate; BMC, bone marrow concentrate; BMMNCs, bone marrow mononuclear cells; BMP-2, bone morphogenetic protein-2; BMSCs, bone marrow-derived mesenchymal stem cells; CD, Core decompression; CPC, calcium phosphate; CSS, cap-shaped separation; Cell implantation; Cell therapy; DBM, demineralized bone matrix; Femoral head; HHS, Harris hip score; IP-CHA, interconnected porous calcium hydroxyapatite; MRI, magnetic resonance imaging; MSCs, Mesenchymal stem cells; MVD, microvessel density; ONFH, Osteonecrosis of the femoral head; Osteonecrosis; PBMSCs, peripheral blood-derived MSCs; PLGA, poly lactide-co-glycolide; RCT, randomized controlled trial; SCPP, strontium-doped calcium polyphosphate; SVF, stromal vascular fractions; Stem cells; THA, total hip arthroplasty; TMCs, transformed mesenchymal cells; TNF, tumor necrosis factor; Tissue engineering; UCMSCs, umbilical cord-derived mesenchymal stem cells; VAS, visual analogue scale; VEGF, vascular endothelial growth factor; WOMAC, Western Ontario and McMaster Universities Arthritis Index; XACB, xenogeneic antigen-extracted cancellous bone; bFGF, basic fibroblast growth factor; β-TCP, beta-tricalcium phosphate.
© 2020 The Japanese Society for Regenerative Medicine. Production and hosting by Elsevier B.V.
Conflict of interest statement
The authors declare that they have no conflict of interest to this study.
Figures



References
-
- Feng Y., Yang S.H., Xiao B.J., Xu W.H., Ye S.N., Xia T. Decreased in the number and function of circulation endothelial progenitor cells in patients with avascular necrosis of the femoral head. Bone. 2010;46:32–40. - PubMed
-
- Mont M.A., Hungerford D.S. Non-traumatic avascular necrosis of the femoral head. J Bone Joint Surg Am. 1995;77:459–474. - PubMed
-
- Gardeniers J.W.M. Treatment of osteonecrosis by joint replacement. Hip Int. 1998;8:159–166.
-
- Radl R., Hungerford M., Materna W., Rehak P., Windhager R. High failure rate and stem migration of an uncemented femoral component in patients with femoral head osteonecrosis than in patients with osteoarthrosis. Acta Orthop. 2005;76:49–55. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

