Controlled dispersion of ZnO nanoparticles produced by basic precipitation in solvothermal processes
- PMID: 33426331
- PMCID: PMC7779710
- DOI: 10.1016/j.heliyon.2020.e05821
Controlled dispersion of ZnO nanoparticles produced by basic precipitation in solvothermal processes
Abstract
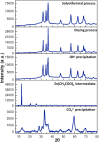
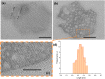
Zinc oxide nanoparticles were successfully synthesized under precipitation processes, using ZnSO4·7H2O as a Zn2+ precursor and K2CO3 used as a basic source, and hydrozincite was obtained as an intermediary, which was treated under two procedures; first procedure involved multiple stages to get final precipitated with NaOH, and in the second procedure the hydrozincite was straightforwardly dried at 220 °C. By both processes ZnO structures were obtained, which were turned into nanoparticles by a solvothermal treatment, for four hours in ethylene glycol at 200 °C. The final products for the first procedure was conglomerate of spherical nanoparticles with sizes ranged between 5-10 nm and dispersed ellipsoidal nanoparticles for the second procedure. Apart off the two procedures mentioned above, another synthesis was carried out with the same Zn2+ precursor but now using NaOH, and the solvothermal treatment produced ZnO mixed micro-structures which under ultrasonic cavitation disaggregated on mesoporous ZnO nanoplates of hexagonal shapes with nanopore sizes of approximately 0.35 nm. All ZnOs synthesized were structurally characterized with XRD, TEM and FT-IR techniques, and electronically with UV-Vis absorption and diffuse reflectance spectroscopies.
Keywords: Alkaline media; Precipitation; Solvothermal treatment; Ultrasonic cavitation; Zinc oxide nanoparticles; ZnO mesoporous.
© 2020 The Authors. Published by Elsevier Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Ameri M., Raoufi M., Zamani-Meymian M.R., Samavat F., Fathollahi M.R., Mohajerani E. Self-assembled ZnO nanosheet-based spherical structure as photoanode in dye-sensitized solar cells. J. Electron. Mater. 2018;47:1993–1999.
-
- Haq A.N.U., Nadhman A., Ullah I., Mustafa G., Yasinzai M., Khan I. Synthesis approaches of zinc oxide nanoparticles: the dilemma of ecotoxicity. J. Nanomater. 2017;2017:8510342.
-
- Kwak J., Bae W.K., Lee D., Park I., Lim J., Park M., Cho H., Woo H., Yoon D.Y., Char K., Lee S., Lee C. Bright and efficient full-color colloidal quantum dot light-emitting diodes using an inverted device structure. Nano Lett. 2012;12:2362–2366. - PubMed
-
- Marcantonio M.D., Gellner S., Namanga J.E., Frohleiks J., Gerlitzki N., Vollkommer F., Bacher G., Nannen E. Performance enhancement by ZnO nanoparticle layer in hybrid ionic transition metal complex-light-emitting electrochemical cells (iTMC-LECs) Adv. Mater. Technol. 2017;2:1600215.
-
- Qian L., Zheng Y., Choudhury K.R., Bera D., So F., Xue J., Holloway P.H. Electroluminescence from light-emitting polymer/ZnO nanoparticle heterojunctions at sub-bandgap voltages. Nano Today. 2010;5:384–389.
Publication types
LinkOut - more resources
Full Text Sources

