The application of nanoparticles in cancer immunotherapy: Targeting tumor microenvironment
- PMID: 33426371
- PMCID: PMC7773537
- DOI: 10.1016/j.bioactmat.2020.12.010
The application of nanoparticles in cancer immunotherapy: Targeting tumor microenvironment
Abstract
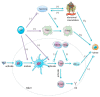
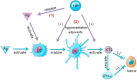
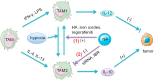
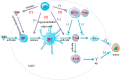
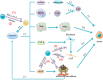
The tumor development and metastasis are closely related to the structure and function of the tumor microenvironment (TME). Recently, TME modulation strategies have attracted much attention in cancer immunotherapy. Despite the preliminary success of immunotherapeutic agents, their therapeutic effects have been restricted by the limited retention time of drugs in TME. Compared with traditional delivery systems, nanoparticles with unique physical properties and elaborate design can efficiently penetrate TME and specifically deliver to the major components in TME. In this review, we briefly introduce the substitutes of TME including dendritic cells, macrophages, fibroblasts, tumor vasculature, tumor-draining lymph nodes and hypoxic state, then review various nanoparticles targeting these components and their applications in tumor therapy. In addition, nanoparticles could be combined with other therapies, including chemotherapy, radiotherapy, and photodynamic therapy, however, the nanoplatform delivery system may not be effective in all types of tumors due to the heterogeneity of different tumors and individuals. The changes of TME at various stages during tumor development are required to be further elucidated so that more individualized nanoplatforms could be designed.
Keywords: AC-NPs, antigen-capturing nanoparticles; ANG2, angiopoietin-2; APCs, antigen-presenting cells; Ab, antibodies; Ag, antigen; AuNCs, gold nanocages; AuNPs, gold nanoparticles; BBB, blood-brain barrier; BTK, Bruton's tyrosine kinase; Bcl-2, B-cell lymphoma 2; CAFs, cancer associated fibroblasts; CAP, cleavable amphiphilic peptide; CAR-T, Chimeric antigen receptor-modified T-cell therapy; CCL, chemoattractant chemokines ligand; CTL, cytotoxic T lymphocytes; CTLA4, cytotoxic lymphocyte antigen 4; CaCO3, calcium carbonate; Cancer immunotherapy; DCs, dendritic cells; DMMA, 2,3-dimethylmaleic anhydrid; DMXAA, 5,6-dimethylxanthenone-4-acetic acid; DSF/Cu, disulfiram/copper; ECM, extracellular matrix; EGFR, epidermal growth factor receptor; EMT, epithelial-mesenchymal transition; EPG, egg phosphatidylglycerol; EPR, enhanced permeability and retention; FAP, fibroblast activation protein; FDA, the Food and Drug Administration; HA, hyaluronic acid; HB-GFs, heparin-binding growth factors; HIF, hypoxia-inducible factor; HPMA, N-(2-hydroxypropyl) methacrylamide; HSA, human serum albumin; Hypoxia; IBR, Ibrutinib; IFN-γ, interferon-γ; IFP, interstitial fluid pressure; IL, interleukin; LMWH, low molecular weight heparin; LPS, lipopolysaccharide; M2NP, M2-like TAM dual-targeting nanoparticle; MCMC, mannosylated carboxymethyl chitosan; MDSCs, myeloid-derived suppressor cells; MPs, microparticles; MnO2, manganese dioxide; NF-κB, nuclear factor κB; NK, nature killer; NO, nitric oxide; NPs, nanoparticles; Nanoparticles; ODN, oligodeoxynucleotides; PD-1, programmed cell death protein 1; PDT, photodynamic therapy; PFC, perfluorocarbon; PHDs, prolyl hydroxylases; PLGA, poly(lactic-co-glycolic acid); PS, photosensitizer; PSCs, pancreatic stellate cells; PTX, paclitaxel; RBC, red-blood-cell; RLX, relaxin-2; ROS, reactive oxygen species; SA, sialic acid; SPARC, secreted protein acidic and rich in cysteine; TAAs, tumor-associated antigens; TAMs, tumor-associated macrophages; TDPA, tumor-derived protein antigens; TGF-β, transforming growth factor β; TIE2, tyrosine kinase with immunoglobulin and epidermal growth factor homology domain 2; TIM-3, T cell immunoglobulin domain and mucin domain-3; TLR, Toll-like receptor; TME, tumor microenvironment; TNF-α, tumor necrosis factor alpha; TfR, transferrin receptor; Tregs, regulatory T cells; Tumor microenvironment; UPS-NP, ultra-pH-sensitive nanoparticle; VDA, vasculature disrupting agent; VEGF, vascular endothelial growth factor; cDCs, conventional dendritic cells; melittin-NP, melittin-lipid nanoparticle; nMOFs, nanoscale metal-organic frameworks; scFv, single-chain variable fragment; siRNA, small interfering RNA; tdLNs, tumor-draining lymph nodes; α-SMA, alpha-smooth muscle actin.
© 2020 [The Author/The Authors].
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- Wu T., Dai Y. Tumor microenvironment and therapeutic response. Canc. Lett. 2017;387:61–68. - PubMed
-
- Denton A.E., Roberts E.W., Fearon D.T. Stromal cells in the tumor microenvironment. Adv. Exp. Med. Biol. 2018;1060:99–114. - PubMed
-
- Musetti S., Huang L. Nanoparticle-mediated remodeling of the tumor microenvironment to enhance immunotherapy. ACS Nano. 2018;12:11740–11755. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

