Efficacy and Safety of PCSK9 Inhibitors in Hypercholesterolemia Associated With Refractory Nephrotic Syndrome
- PMID: 33426389
- PMCID: PMC7783565
- DOI: 10.1016/j.ekir.2020.09.046
Efficacy and Safety of PCSK9 Inhibitors in Hypercholesterolemia Associated With Refractory Nephrotic Syndrome
Abstract
Introduction: Treatment of hypercholesterolemia in refractory nephrotic syndrome remains a therapeutic challenge. There is not enough evidence supporting the efficacy of statins, and these drugs can be associated with an increased incidence of adverse effects. Herein we summarize our clinical experience with 12 patients suffering from refractory nephrotic syndrome with associated vascular disease and uncontrolled hypercholesterolemia despite treatment with statins who were treated with proprotein convertase subtilisin kexin 9 (PCSK9) inhibitors.
Methods: Twelve adult patients with primary nephrotic syndrome refractory to multiple lines of immunosuppressive treatment who suffered from clinical atheromatous vascular disease were treated with PCSK9 inhibitors according to the prescription guidelines for secondary prevention of cardiovascular events. Eight patients with refractory nephrotic syndrome without vascular disease treated with atorvastatin comprised the control group.
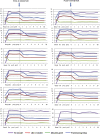
Results: Four weeks after treatment with PCSK9 inhibitors, a statistically significant decrease in total cholesterol and low-density lipoprotein cholesterol (LDL-C) levels was observed without significant changes in serum albumin levels or proteinuria. The mean LDL-C decrease was 36.8% ± 4.9% mmol/L at 4 weeks and remained unchanged throughout the follow-up period. In the control group, there were no significant changes in the levels of total cholesterol or LDL-C during the follow-up period. At the diagnosis of nephrotic syndrome, plasma PCSK9 levels were 334 ± 40 ng/mL and correlated significantly with serum LDL-C levels (r = 0.49, P = 0.023). Six months after starting treatment with PCSK9 inhibitors, plasma PCSK9 levels were significantly reduced to values of 190 ± 36 ng/mL (P = 0.001) with a mean relative reduction of 42.3% ± 12.6%. No local adverse effects were seen at the injection site and no significant changes were seen in the levels of transaminase, creatine phosphokinase, or aldolase.
Conclusion: PCSK9 inhibitors may be an effective and safe alternative for the treatment of hypercholesterolemia associated with refractory nephrotic syndrome.
Keywords: PSCK9 inhibitors; dyslipidemia; hypercholesterolemia; nephrotic syndrome.
© 2020 International Society of Nephrology. Published by Elsevier Inc.
Figures



References
-
- Scandinavian Simvastatin Survival Study Group Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344:1383–1389. - PubMed
-
- Radhakrishnan J., Appel A.S., Valeri A., Appel G.B. The nephrotic syndrome, lipids, and risk factors for cardiovascular disease. Am J Kidney Dis. 1993;22:135–142. - PubMed
-
- Liu S., Vaziri N.D. Role of PCSK9 and IDOL in the pathogenesis of acquired LDL receptor deficiency and hypercholesterolemia in nephrotic syndrome. Nephrol Dial Transplant. 2014;29:538–543. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

