Novel case of androgen receptor-positive cancer of unknown primary without serum prostate-specific antigen elevation that became progression free in the long term after primary combined androgen blockade
- PMID: 33426501
- PMCID: PMC7784734
- DOI: 10.1002/iju5.12241
Novel case of androgen receptor-positive cancer of unknown primary without serum prostate-specific antigen elevation that became progression free in the long term after primary combined androgen blockade
Abstract
Introduction: The prognosis of cancer of unknown primary is very poor. Such a prognosis can be improved by characterizing primary characteristics and developing tailored site-specific therapy, especially for androgen receptor-positive adenocarcinoma. However, in such cases without elevated prostate-specific antigen, the efficacy of androgen deprivation therapy is unclear.
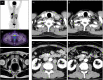
Case presentation: Herein, we report a case that presented with a retroperitoneal cancer of unknown primary that was confirmed as an androgen receptor-positive adenocarcinoma without prostate-specific antigen elevation. Pelvic magnetic resonance imaging did not reveal any suspicious cancer lesions in the prostate. Furthermore, malignant cells were not present in a prostate biopsy specimen. In spite of the prostate-specific antigen level, on the basis of immunohistochemical analyses, including NKX3.1, the patient was first treated with androgen deprivation therapy, leading to long-term progression-free survival.
Conclusion: Early androgen deprivation therapy based on immunohistochemical analyses might lead to a good outcome in androgen receptor-positive adenocarcinoma cancer of unknown primary patients regardless of prostate-specific antigen level.
Keywords: NKX3.1; androgen receptor; cancer of unknown primary.
© 2020 The Authors. IJU Case Reports published by John Wiley & Sons Australia, Ltd on behalf of the Japanese Urological Association.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Pavlidis N, Pentheroudakis G. Cancer of unknown primary site:20 questions to be answered. Ann. Oncol. 2010; 21(Suppl 7): 303–7. - PubMed
-
- Ettinger DS, Arnoletti JP, Gockerman JP et al Occult primary cancer clinical practice guidelines. J. Natl Compr. Netw. 2005; 3: 214–33. - PubMed
-
- NCCN Clinical Practice Guidelines in Oncology . Occult Primary (Cancer of unknown primary [CUP] Version 2.2019.
-
- Sebastian M, Anna MC, Sergi S et al Epigenetic profiling to classify of unknown primary: a multicentre, retrospective analysis. Lancet Oncol. 2016; 17: 1386–95. - PubMed
-
- Pentheroudakis G, GolfinopoulosV PN. Switching benchmarks in cancer of unknown primary: from autopsy to microarray. Eur. J. Cancer 2007; 43: 2026–36. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
