Spectral CT quantification stability and accuracy for pediatric patients: A phantom study
- PMID: 33426801
- PMCID: PMC7984483
- DOI: 10.1002/acm2.13161
Spectral CT quantification stability and accuracy for pediatric patients: A phantom study
Abstract
Background: Spectral computed tomography (spectral CT) provides access to clinically relevant measures of endogenous and exogenous materials in patients. For pediatric patients, current spectral CT applications include lesion characterization, quantitative vascular imaging, assessments of tumor response to treatment, and more.
Objective: The aim of this study is a comprehensive investigation of the accuracy and stability of spectral quantifications from a spectral detector-based CT system with respect to different patient sizes and radiation dose levels relevant for the pediatric population.
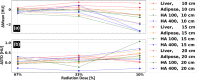
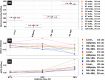
Materials and methods: A spectral CT phantom with tissue-mimicking materials and iodine concentrations relevant for pediatric imaging was scanned on a spectral detector CT system using a standard pediatric abdominal protocol at 100%, 67%, 33% and 10% of the nominal radiation dose level. Different pediatric patient sizes were simulated using supplemental 3D-printed extension rings. Virtual mono-energetic, iodine density, effective atomic number, and electron density results were analyzed for stability with respect to radiation dose and patient size.
Results: Compared to conventional CT imaging, a pronounced improvement in the stability of attenuation measurements across patient size was observed when using virtual mono-energetic images. Iodine densities were within 0.1 mg/ml, effective atomic numbers were within 0.26 atomic numbers and electron density quantifications were within ±1.0% of their respective nominal values. Relative to the nominal dose clinical protocol, differences in attenuation of all tissue-mimicking materials were maintained below 1.6 HU for a 33% dose reduction, below 2.7 HU for a 67% dose reduction and below 3.7 HU for a 90% dose reduction, for all virtual mono-energetic energies equal to or greater than 50 keV. Iodine, and effective atomic number quantifications were stable to within 0.1 mg/ml and 0.06 atomic numbers, respectively, across all measured dose levels.
Conclusion: Spectral CT provides accurate and stable material quantification with respect to radiation dose reduction (up to 90%) and differing pediatric patient size. The observed consistency is an important step towards quantitative pediatric imaging at low radiation exposure levels.
Keywords: dual energy CT; pediatric imagining; quantitative imaging; spectral CT.
© 2021 The Authors. Journal of Applied Clinical Medical Physics published by Wiley Periodicals LLC on behalf of American Association of Physicists in Medicine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Alvarez RE, Macovski A. Energy‐selective reconstructions in X‐ray computerised tomography. Phys Med Biol. 1976;21:002. - PubMed
-
- Flohr TG, McCollough CH, Bruder H, et al. First performance evaluation of a dual‐source CT (DSCT) system. Eur Radiol. 2006;16:256–268. - PubMed
-
- Caruso D, De Cecco CN, Schoepf UJ, et al. Can dual‐energy computed tomography improve visualization of hypoenhancing liver lesions in portal venous phase? Assessment of advanced image‐based virtual monoenergetic images. Clin Imaging. 2017;41:118–124. - PubMed
-
- Wang Q, Shi G, Qi X, Fan X, Wang L. Quantitative analysis of the dual‐energy CT virtual spectral curve for focal liver lesions characterization. Eur J Radiol. 2014;83:1759–1764. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

