Questions and answers on iron deficiency treatment selection and the use of intravenous iron in routine clinical practice
- PMID: 33426933
- PMCID: PMC7877947
- DOI: 10.1080/07853890.2020.1867323
Questions and answers on iron deficiency treatment selection and the use of intravenous iron in routine clinical practice
Abstract
Iron deficiency is a common cause of morbidity and can arise as a consequence or complication from many diseases. The use of intravenous iron has increased significantly in the last decade, but concerns remain about indications and administration. Modern intravenous iron preparations can facilitate rapid iron repletion in one or two doses, both for absolute iron deficiency and, in the presence of inflammation, functional iron deficiency, where oral iron therapy is ineffective or has not worked. A multidisciplinary team of experts experienced in iron deficiency undertook a consensus review to support healthcare professionals with practical advice on managing iron deficiency in gastrointestinal, renal and cardiac disease, as well as; pregnancy, heavy menstrual bleeding, and surgery. We explain how intravenous iron may work where oral iron has not. We provide context on how and when intravenous iron should be administered, and informed opinion on potential benefits balanced with potential side-effects. We propose how intravenous iron side-effects can be anticipated in terms of what they may be and when they may occur. The aim of this consensus is to provide a practical basis for educating and preparing staff and patients on when and how iron infusions can be administered safely and efficiently. Key messages Iron deficiency treatment selection is driven by several factors, including the presence of inflammation, the time available for iron replenishment, and the anticipated risk of side-effects or intolerance. Intravenous iron preparations are indicated for the treatment of iron deficiency when oral preparations are ineffective or cannot be used, and therefore have applicability in a wide range of clinical contexts, including chronic inflammatory conditions, perioperative settings, and disorders associated with chronic blood loss. Adverse events occurring with intravenous iron can be anticipated according to when they typically occur, which provides a basis for educating and preparing staff and patients on how iron infusions can be administered safely and efficiently.
Keywords: Anaemia; cardiovascular diseases; chronic; erythrocyte transfusion; inflammatory bowel diseases; infusions; intravenous; iron; iron-deficiency; menorrhagia; pregnancy complications; renal insufficiency.
Conflict of interest statement
Outside of this submitted work, TR reports grants, personal fees and non-financial support from Pharmocosmos and Vifor Pharma, grants and personal fees from Acelity, and personal fees from Amgen, Medtronic, and TIASH. TR is a director of The Iron Clinic Ltd and Veincare London Ltd, and vascular lead for 18 Week Support Ltd. CB reports personal fees from Pharmacosmos and Vifor Pharma. MJB reports grants and personal fees from Vifor Pharma and Tillotts Pharma. SL reports personal fees from Pharmacosmos and Vifor Pharma. ICM reports personal fees from Vifor Pharma. LPM reports non-financial support from Vifor Pharma. MGM reports personal fees from Vifor Pharma, Daiichi Sankyo, and American Regent. EN reports stock ownership of Intrinsic LifeSciences and Silarus Therapeutics, and consulting for Vifor Pharma, Ionis Pharmaceuticals, and Protagonist Therapeutics. GMCR has no disclosures to report. IS reports personal fees from Vifor Pharma. GW reports personal fees from Vifor Pharma. No Data Availability Statement for the manuscript.
Figures
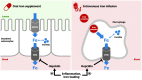

References
-
- Camaschella C. Iron-deficiency anemia. N Engl J Med. 2015;372:1832–1843. - PubMed
-
- Cheong Y, Cameron IT, Critchley HOD.. Abnormal uterine bleeding. Br Med Bull. 2019;131:119. - PubMed
-
- Forbes A, Escher J, Hébuterne X, et al. . ESPEN guideline: clinical nutrition in inflammatory bowel disease. Clin Nutr. 2017;36:321–347. - PubMed
-
- Kidney Disease: Improving Global Outcomes (KDIGO) Anemia Work Group. KDIGO Clinical Practice Guideline for Anemia in Chronic Kidney Disease. Kidney Int (Suppl). 2012;2:279–335.
-
- Ponikowski P, Voors AA, Anker SD, Bueno H, et al. , ESC Scientific Document Group . 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
