Photothermal therapies to improve immune checkpoint blockade for cancer
- PMID: 33426992
- PMCID: PMC7808273
- DOI: 10.1080/02656736.2020.1797190
Photothermal therapies to improve immune checkpoint blockade for cancer
Abstract
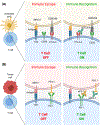
Immune checkpoint blockade (ICB) comprising monoclonal antibodies (mAbs) against immune 'checkpoints', such as CTLA-4 and the PD1/PDL1 axis have dramatically improved clinical outcomes for patients with cancer. However, ICB by itself has failed to provide benefit in a wide range of solid tumors, where recurrence still occurs with high incidence. These poor response rates may be due to the therapeutic shortcomings of ICB; namely, a lack of cancer-specific cytotoxicity and ability to debulk tumors. To overcome these limitations, effective ICB therapy may require the combination with other complementary therapeutic platforms. Here, we propose that photothermal therapy (PTT) is an ideal therapeutic modality for combination with ICB because it can generate both tumor-specific cytotoxicity and immunogenicity. PTT elicits these specific effects because it is a localized thermal ablation technique that utilizes light-responsive nanoparticles activated by a wavelength-matched laser. While ICB immunotherapy alone improves cancer immunogenicity but does not generate robust antitumor cytotoxicity, nanoparticle-based PTT elicits targeted and controlled cytotoxicity but sub-optimal long-term immunogenicity. Thus, the two platforms offer complementary and potentially synergistic antitumor effects, which will be detailed in this review. We highlight three classes of nanoparticles used as agents of PTT (i.e., metallic inorganic nanoparticles, carbon-based nanoparticles and organic dyes), and illustrate the potential for nanoparticle-based PTT to potentiate the effects of ICB in preclinical models. Through this discussion, we aim to present PTT combined with ICB as a potent synergistic combination treatment for diverse cancer types currently refractory to ICB as well as PTT monotherapies.
Keywords: Photothermal therapy; cancer; immune checkpoint blockade; immunotherapy; nanoparticle; thermal ablation.
Conflict of interest statement
Declaration of Interest
The authors report no conflicts of interest
Figures

References
-
- Martins GA, Tadokoro CE, Silva RB, Silva JS, Rizzo LV. CTLA-4 Blockage Increases Resistance to Infection with the Intracellular Protozoan Trypanosoma cruzi. The Journal of Immunology. 2004;172(8):4893. - PubMed
-
- Ariyan C, Salvalaggio P, Fecteau S, Deng S, Rogozinski L, Mandelbrot D, et al. Cutting Edge: Transplantation Tolerance through Enhanced CTLA-4 Expression. The Journal of Immunology. 2003;171(11):5673. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
