Systemically delivered antibody-labeled magnetic iron oxide nanoparticles are less toxic than plain nanoparticles when activated by alternating magnetic fields
- PMID: 33426997
- PMCID: PMC7810240
- DOI: 10.1080/02656736.2020.1776901
Systemically delivered antibody-labeled magnetic iron oxide nanoparticles are less toxic than plain nanoparticles when activated by alternating magnetic fields
Abstract
Objective: Toxicity from off-target heating with magnetic hyperthermia (MHT) is generally assumed to be understood. MHT research focuses on development of more potent heating magnetic iron oxide nanoparticles (MIONs), yet our understanding of factors that define biodistribution following systemic delivery remains limited. Preclinical development relies on mouse models, thus understanding off-target heating with MHT in mice provides critical knowledge for clinical development.
Methods: Eight-week old female nude mice received a single tail vein injection of bionized nanoferrite (BNF) MIONs or a counterpart labeled with a polyclonal human antibody (BNF-IgG) at 1 mg, 3 mg or 5 mg Fe/mouse on day 1. On day 3, mice were exposed to an alternating magnetic field (AMF) having amplitude of 32, 48 or 64 kA/m at ∼145 kHz for 20 min. Twenty-four hours later, blood, livers and spleens were harvested and analyzed.
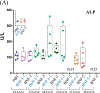
Results: Damage to livers was apparent by histology and serum liver enzymes following MHT with BNF or BNF-IgG at doses ≥3 mg Fe and AMF amplitudes ≥48 kA/m. Differences between effects with BNF vs. BNF-IgG at a dose of 3 mg Fe were noted in all measures, with less damage and increased survival occurring in mice injected with BNF-IgG. Necropsies revealed severe damage to duodenum and upper small intestines, likely the immediate cause of death at the highest MHT doses.
Conclusion: Results demonstrate that the MION coating affects biodistribution, which in turn determines off-target effects. Developments to improve heating capabilities of MIONs may be clinically irrelevant without better control of biodistribution.
Keywords: Iron oxide nanoparticles; alternating magnetic fields; hyperthermia; magnetic nanoparticles; monoclonal antibody.
Figures















Similar articles
-
Characterization of intratumor magnetic nanoparticle distribution and heating in a rat model of metastatic spine disease.J Neurosurg Spine. 2014 Jun;20(6):740-50. doi: 10.3171/2014.2.SPINE13142. Epub 2014 Apr 4. J Neurosurg Spine. 2014. PMID: 24702509
-
Hyperthermia generated by magnetic nanoparticles for effective treatment of disseminated peritoneal cancer in an orthotopic nude-mouse model.Cell Cycle. 2021 Jun;20(12):1122-1133. doi: 10.1080/15384101.2021.1919441. Epub 2021 Jun 10. Cell Cycle. 2021. PMID: 34110969 Free PMC article.
-
Image-guided thermal therapy with a dual-contrast magnetic nanoparticle formulation: A feasibility study.Int J Hyperthermia. 2016 Aug;32(5):543-57. doi: 10.3109/02656736.2016.1159737. Epub 2016 May 5. Int J Hyperthermia. 2016. PMID: 27151045 Free PMC article.
-
Magnetic nanoparticles and clusters for magnetic hyperthermia: optimizing their heat performance and developing combinatorial therapies to tackle cancer.Chem Soc Rev. 2021 Oct 18;50(20):11614-11667. doi: 10.1039/d1cs00427a. Chem Soc Rev. 2021. PMID: 34661212 Review.
-
Enhancement of CD8+ T-Cell-Mediated Tumor Immunotherapy via Magnetic Hyperthermia.ChemMedChem. 2022 Jan 19;17(2):e202100656. doi: 10.1002/cmdc.202100656. Epub 2021 Dec 4. ChemMedChem. 2022. PMID: 34806311 Review.
Cited by
-
HYPER: pre-clinical device for spatially-confined magnetic particle hyperthermia.Int J Hyperthermia. 2023;40(1):2272067. doi: 10.1080/02656736.2023.2272067. Epub 2023 Oct 24. Int J Hyperthermia. 2023. PMID: 37875265 Free PMC article.
-
Clinical magnetic hyperthermia requires integrated magnetic particle imaging.Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2022 May;14(3):e1779. doi: 10.1002/wnan.1779. Epub 2022 Mar 3. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2022. PMID: 35238181 Free PMC article. Review.
References
-
- Weinstein JS, Varallyay CG, Dosa E, Gahramanov S, Hamilton B, Rooney WD, Muldoon LL & Neuwelt EA Superparamagnetic iron oxide nanoparticles: diagnostic magnetic resonance imaging and potential therapeutic applications in neurooncology and central nervous system inflammatory pathologies, a review. J Cereb Blood Flow Metab, 30, 15–35 (2010). - PMC - PubMed
-
- Ivkov R Magnetic nanoparticle hyperthermia: A new frontier in biology and medicine? Taylor & Francis, 29(8), 703–705 (2013). - PubMed
-
- Kandasamy G & Maity D Recent advances in superparamagnetic iron oxide nanoparticles (SPIONs) for in vitro and in vivo cancer nanotheranostics. Int J Pharm, 496, 191–218 (2015). - PubMed
-
- Theil EC Iron, ferritin, and nutrition. Annual Review of Nutrition, 24, 327–343 (2004). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
