Stochastic asymmetric repartition of lytic machinery in dividing CD8+ T cells generates heterogeneous killing behavior
- PMID: 33427199
- PMCID: PMC7867409
- DOI: 10.7554/eLife.62691
Stochastic asymmetric repartition of lytic machinery in dividing CD8+ T cells generates heterogeneous killing behavior
Abstract
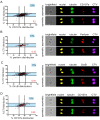
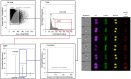
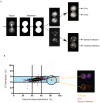
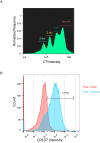
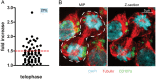
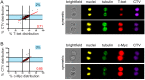
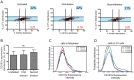
Cytotoxic immune cells are endowed with a high degree of heterogeneity in their lytic function, but how this heterogeneity is generated is still an open question. We therefore investigated if human CD8+ T cells could segregate their lytic components during telophase, using imaging flow cytometry, confocal microscopy, and live-cell imaging. We show that CD107a+-intracellular vesicles, perforin, and granzyme B unevenly segregate in a constant fraction of telophasic cells during each division round. Mathematical modeling posits that unequal lytic molecule inheritance by daughter cells results from the random distribution of lytic granules on the two sides of the cleavage furrow. Finally, we establish that the level of lytic compartment in individual cytotoxic T lymphocyte (CTL) dictates CTL killing capacity.
Keywords: cell division; cytotoxic t lymphocytes; human; human lymphocytes; immunology; inflammation; lysosomal-associated membrane proteins; lytic granules.
© 2021, Lafouresse et al.
Conflict of interest statement
FL, RJ, SM, MD, VD, EE, MP, SG, SV No competing interests declared
Figures
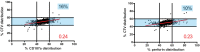




Similar articles
-
Serial killing by cytotoxic T lymphocytes: T cell receptor triggers degranulation, re-filling of the lytic granules and secretion of lytic proteins via a non-granule pathway.Eur J Immunol. 1995 Apr;25(4):1071-9. doi: 10.1002/eji.1830250432. Eur J Immunol. 1995. PMID: 7737276
-
Stepwise maturation of lytic granules during differentiation and activation of human CD8+ T lymphocytes.PLoS One. 2011;6(11):e27057. doi: 10.1371/journal.pone.0027057. Epub 2011 Nov 4. PLoS One. 2011. PMID: 22073254 Free PMC article.
-
Induction of granzyme B and T cell cytotoxic capacity by IL-2 or IL-15 without antigens: multiclonal responses that are extremely lytic if triggered and short-lived after cytokine withdrawal.Cytokine. 2006 Nov;36(3-4):148-59. doi: 10.1016/j.cyto.2006.11.008. Epub 2006 Dec 22. Cytokine. 2006. PMID: 17188506 Free PMC article.
-
Activation of primary T lymphocytes results in lysosome development and polarized granule exocytosis in CD4+ and CD8+ subsets, whereas expression of lytic molecules confers cytotoxicity to CD8+ T cells.J Leukoc Biol. 2006 Oct;80(4):827-37. doi: 10.1189/jlb.0603298. Epub 2006 Aug 4. J Leukoc Biol. 2006. PMID: 16891618
-
Signals Controlling Lytic Granule Polarization at the Cytotoxic Immune Synapse.Front Immunol. 2018 Feb 20;9:307. doi: 10.3389/fimmu.2018.00307. eCollection 2018. Front Immunol. 2018. PMID: 29515593 Free PMC article. Review.
Cited by
-
They Might Cut It-Lysosomes and Autophagy in Mitotic Progression.Front Cell Dev Biol. 2021 Aug 13;9:727538. doi: 10.3389/fcell.2021.727538. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34485308 Free PMC article. Review.
-
Isolating and targeting a highly active, stochastic dendritic cell subpopulation for improved immune responses.Cell Rep. 2022 Nov 1;41(5):111563. doi: 10.1016/j.celrep.2022.111563. Cell Rep. 2022. PMID: 36323246 Free PMC article.
-
Synapse-tuned CARs enhance immune cell anti-tumor activity.Nat Biotechnol. 2023 Oct;41(10):1434-1445. doi: 10.1038/s41587-022-01650-2. Epub 2023 Feb 2. Nat Biotechnol. 2023. PMID: 36732477 Free PMC article.
-
Asymmetric T-cell division: insights from cutting-edge experimental techniques and implications for immunotherapy.Front Immunol. 2024 Mar 1;15:1301378. doi: 10.3389/fimmu.2024.1301378. eCollection 2024. Front Immunol. 2024. PMID: 38495874 Free PMC article. Review.
-
Greek Fire, Poison Arrows, and Scorpion Bombs: How Tumor Cells Defend Against the Siege Weapons of Cytotoxic T Lymphocytes.Front Immunol. 2022 May 3;13:894306. doi: 10.3389/fimmu.2022.894306. eCollection 2022. Front Immunol. 2022. PMID: 35592329 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

