Antibodies Contributing to Focal Epilepsy Signs and Symptoms Score
- PMID: 33427313
- PMCID: PMC8048471
- DOI: 10.1002/ana.26013
Antibodies Contributing to Focal Epilepsy Signs and Symptoms Score
Abstract
Objective: Diagnosing autoimmune encephalitis (AIE) is difficult in patients with less fulminant diseases such as epilepsy. However, recognition is important, as patients require immunotherapy. This study aims to identify antibodies in patients with focal epilepsy of unknown etiology, and to create a score to preselect patients requiring testing.
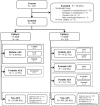
Methods: In this prospective, multicenter cohort study, adults with focal epilepsy of unknown etiology, without recognized AIE, were included, between December 2014 and December 2017, and followed for 1 year. Serum, and if available cerebrospinal fluid, were analyzed using different laboratory techniques. The ACES score was created using factors favoring an autoimmune etiology of seizures (AES), as determined by multivariate logistic regression. The model was externally validated and evaluated using the Concordance (C) statistic.
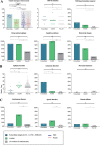
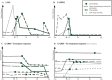
Results: We included 582 patients, with median epilepsy duration of 8 years (interquartile range = 2-18). Twenty patients (3.4%) had AES, of whom 3 had anti-leucine-rich glioma inactivated 1, 3 had anti-contactin-associated protein-like 2, 1 had anti-N-methyl-D-aspartate receptor, and 13 had anti-glutamic acid decarboxylase 65 (enzyme-linked immunosorbent assay concentrations >10,000IU/ml). Risk factors for AES were temporal magnetic resonance imaging hyperintensities (odds ratio [OR] = 255.3, 95% confidence interval [CI] = 19.6-3332.2, p < 0.0001), autoimmune diseases (OR = 13.31, 95% CI = 3.1-56.6, p = 0.0005), behavioral changes (OR 12.3, 95% CI = 3.2-49.9, p = 0.0003), autonomic symptoms (OR = 13.3, 95% CI = 3.1-56.6, p = 0.0005), cognitive symptoms (OR = 30.6, 95% CI = 2.4-382.7, p = 0.009), and speech problems (OR = 9.6, 95% CI = 2.0-46.7, p = 0.005). The internally validated C statistic was 0.95, and 0.92 in the validation cohort (n = 128). Assigning each factor 1 point, an antibodies contributing to focal epilepsy signs and symptoms (ACES) score ≥ 2 had a sensitivity of 100% to detect AES, and a specificity of 84.9%.
Interpretation: Specific signs point toward AES in focal epilepsy of unknown etiology. The ACES score (cutoff ≥ 2) is useful to select patients requiring antibody testing. ANN NEUROL 2021;89:698-710.
© 2021 The Authors. Annals of Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
Nothing to report.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

