Preclinical stem cell therapy in fetuses with myelomeningocele: A systematic review and meta-analysis
- PMID: 33427329
- PMCID: PMC7611444
- DOI: 10.1002/pd.5887
Preclinical stem cell therapy in fetuses with myelomeningocele: A systematic review and meta-analysis
Abstract
Objective: We performed a systematic review to summarize the efficacy and safety of in utero stem cells application in preclinical models with myelomeningocele (MMC).
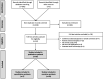
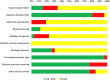
Methods: The study was registered with PROSPERO (CRD42019160399). We searched MEDLINE, Embase, Web of Science, Scopus and CENTRAL for publications articles on stem cell therapy in animal fetuses with MMC until May 2020. Publication quality was assessed by the SYRCLE's tool. Meta-analyses were pooled if studies were done in the same animal model providing similar type of stem cell used and outcome measurements. Narrative synthesis was performed for studies that could not be pooled.
Results: Nineteen and seven studies were included in narrative and quantitative syntheses, respectively. Most used mesenchymal stem cells (MSCs) and primarily involved ovine and rodent models. Both intra-amniotic injection of allogeneic amniotic fluid (AF)-MSCs in rat MMC model and the application of human placental (P)-MSCs to the spinal cord during fetal surgery in MMC ovine model did not compromise fetal survival rates at term (rat model, relative risk [RR] 1.03, 95% CI 0.92-1.16; ovine model, RR 0.94, 95% CI 0.78-1.13). A single intra-amniotic injection of allogeneic AF-MSCs into rat MMC model was associated with a higher rate of complete defect coverage compared to saline injection (RR 16.35, 95% CI 3.27-81.79). The incorporation of human P-MSCs as a therapeutic adjunct to fetal surgery in the ovine MMC model significantly improved sheep locomotor rating scale after birth (mean difference 5.18, 95% CI 3.36-6.99).
Conclusions: Stem cell application during prenatal period in preclinical animal models is safe and effective.
© 2021 The Authors. Prenatal Diagnosis published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures

Similar articles
-
Safety and efficacy of human umbilical cord-derived mesenchymal stromal cells in fetal ovine myelomeningocele repair.Stem Cell Res Ther. 2024 Nov 20;15(1):444. doi: 10.1186/s13287-024-03991-y. Stem Cell Res Ther. 2024. PMID: 39568021 Free PMC article.
-
Placental mesenchymal stromal cells rescue ambulation in ovine myelomeningocele.Stem Cells Transl Med. 2015 Jun;4(6):659-69. doi: 10.5966/sctm.2014-0296. Epub 2015 Apr 24. Stem Cells Transl Med. 2015. PMID: 25911465 Free PMC article.
-
Cell therapy for prenatal repair of myelomeningocele: A systematic review.Curr Res Transl Med. 2020 Nov;68(4):183-189. doi: 10.1016/j.retram.2020.04.004. Epub 2020 Jul 2. Curr Res Transl Med. 2020. PMID: 32624428
-
Biodistribution of allogenic umbilical cord-derived mesenchymal stromal cells after fetal repair of myelomeningocele in an ovine model.Stem Cell Res Ther. 2022 Jul 15;13(1):300. doi: 10.1186/s13287-022-02991-0. Stem Cell Res Ther. 2022. PMID: 35841029 Free PMC article.
-
Human Amniotic Fluid Stem Cells: Therapeutic Potential for Perinatal Patients with Intractable Neurological Disease.Keio J Med. 2018 Dec 26;67(4):57-66. doi: 10.2302/kjm.2017-0019-IR. Epub 2018 Mar 6. Keio J Med. 2018. PMID: 29515049 Review.
Cited by
-
Outcomes of autologous bone marrow mononuclear cell administration in the treatment of neurologic sequelae in children with spina bifida.Stem Cell Res Ther. 2023 Apr 28;14(1):115. doi: 10.1186/s13287-023-03349-w. Stem Cell Res Ther. 2023. PMID: 37118832 Free PMC article.
-
Trends in research related to fetal therapy from 2012 to 2022: a bibliometric analysis.Front Pediatr. 2024 Jan 4;11:1288660. doi: 10.3389/fped.2023.1288660. eCollection 2023. Front Pediatr. 2024. PMID: 38293659 Free PMC article.
-
Safety and efficacy of human umbilical cord-derived mesenchymal stromal cells in fetal ovine myelomeningocele repair.Stem Cell Res Ther. 2024 Nov 20;15(1):444. doi: 10.1186/s13287-024-03991-y. Stem Cell Res Ther. 2024. PMID: 39568021 Free PMC article.
-
Fetal Surgery for Myelomeningocele: Neurosurgical Perspectives.Adv Tech Stand Neurosurg. 2023;47:25-48. doi: 10.1007/978-3-031-34981-2_2. Adv Tech Stand Neurosurg. 2023. PMID: 37640871
-
Regenerative medicine in Obstetrics & Gynecology: Current status under the Act on the Safety of Regenerative Medicine in Japan.Regen Ther. 2024 Aug 13;26:564-570. doi: 10.1016/j.reth.2024.08.003. eCollection 2024 Jun. Regen Ther. 2024. PMID: 39228904 Free PMC article. Review.
References
-
- Syngelaki A, Hammami A, Bower S, Zidere V, Akolekar R, Nicolaides KH. Diagnosis of fetal non‐chromosomal abnormalities on routine ultrasound examination at 11‐13 weeks' gestation. Ultrasound Obstet Gynecol. 2019;54(4):468‐476. - PubMed
-
- Ovaere C, Eggink A, Richter J, et al. Prenatal diagnosis and patient preferences in patients with neural tube defects around the advent of fetal surgery in Belgium and Holland. Fetal Diagn Ther. 2015;37(3):226‐234. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

