Simulating drug penetration during hyperthermic intraperitoneal chemotherapy
- PMID: 33427507
- PMCID: PMC7808385
- DOI: 10.1080/10717544.2020.1862364
Simulating drug penetration during hyperthermic intraperitoneal chemotherapy
Abstract
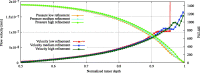
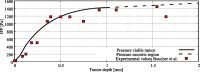
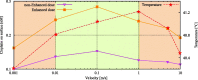
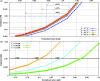
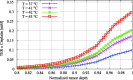
Hyperthermic intraperitoneal chemotherapy (HIPEC) is administered to treat residual microscopic disease after debulking cytoreductive surgery. During HIPEC, a limited number of catheters are used to administer and drain fluid containing chemotherapy (41-43 °C), yielding heterogeneities in the peritoneum. Large heterogeneities may lead to undertreated areas, increasing the risk of recurrences. Aiming at intra-abdominal homogeneity is therefore essential to fully exploit the potential of HIPEC. More insight is needed into the extent of the heterogeneities during treatments and assess their effects on the efficacy of HIPEC. To that end we developed a computational model containing embedded tumor nodules in an environment mimicking peritoneal conditions. Tumor- and treatment-specific parameters affecting drug delivery like tumor size, tumor shape, velocity, temperature and dose were assessed using three-dimensional computational fluid dynamics (CFD) to demonstrate their effect on the drug distribution and accumulation in nodules. Clonogenic assays performed on RKO colorectal cell lines yielded the temperature-dependent IC50 values of cisplatin (19.5-6.8 micromolar for 37-43 °C), used to compare drug distributions in our computational models. Our models underlined that large nodules are more difficult to treat and that temperature and velocity are the most important factors to control the drug delivery. Moderate flow velocities, between 0.01 and 1 m/s, are optimal for the delivery of cisplatin. Furthermore, higher temperatures and higher doses increased the effective penetration depth with 69% and 54%, respectively. We plan to extend the software developed for this study toward patient-specific treatment planning software, capable of mapping and assist in reducing heterogeneous flow patterns.
Keywords: Hyperthermic intrapertioneal chemotherapy (HIPEC); cancer biology; computational fluid dynamics (CFD); computational modeling; drug dynamics; interstitial fluidpressure (IFP); treatment planning software.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures


Similar articles
-
Demonstration of treatment planning software for hyperthermic intraperitoneal chemotherapy in a rat model.Int J Hyperthermia. 2021;38(1):38-54. doi: 10.1080/02656736.2020.1852324. Int J Hyperthermia. 2021. PMID: 33487083
-
The Temperature-Dependent Effectiveness of Platinum-Based Drugs Mitomycin-C and 5-FU during Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in Colorectal Cancer Cell Lines.Cells. 2020 Jul 25;9(8):1775. doi: 10.3390/cells9081775. Cells. 2020. PMID: 32722384 Free PMC article.
-
A 3D CFD model of the interstitial fluid pressure and drug distribution in heterogeneous tumor nodules during intraperitoneal chemotherapy.Drug Deliv. 2019 Dec;26(1):404-415. doi: 10.1080/10717544.2019.1588423. Drug Deliv. 2019. PMID: 30929523 Free PMC article.
-
A guide to establishing a hyperthermic intraperitoneal chemotherapy program in gynecologic oncology.Gynecol Oncol. 2020 Sep;158(3):794-802. doi: 10.1016/j.ygyno.2020.06.487. Epub 2020 Jul 2. Gynecol Oncol. 2020. PMID: 32624234 Review.
-
Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) for colorectal and appendiceal peritoneal metastases - The Hong Kong experience and literature review.Asian J Surg. 2021 Jan;44(1):221-228. doi: 10.1016/j.asjsur.2020.05.010. Epub 2020 Jun 27. Asian J Surg. 2021. PMID: 32605790 Review.
Cited by
-
Advances in the management of peritoneal malignancies.Nat Rev Clin Oncol. 2022 Nov;19(11):698-718. doi: 10.1038/s41571-022-00675-5. Epub 2022 Sep 7. Nat Rev Clin Oncol. 2022. PMID: 36071285 Review.
-
HIPEC in Peritoneal Metastasis of Gastric Origin: A Systematic Review of Regimens and Techniques.J Clin Med. 2022 Mar 7;11(5):1456. doi: 10.3390/jcm11051456. J Clin Med. 2022. PMID: 35268546 Free PMC article. Review.
-
CHIPOR, HORSE, and beyond: unraveling the role of hyperthermic intraperitoneal chemotherapy (HIPEC) in ovarian cancer.J Gynecol Oncol. 2025 Jan;36(1):e73. doi: 10.3802/jgo.2025.36.e73. J Gynecol Oncol. 2025. PMID: 39900348 Free PMC article. No abstract available.
-
Indication of Hyperthermic Intraperitoneal Chemotherapy in Gastric Cancer (Gastripec, Gastrichip).Visc Med. 2022 Apr;38(2):81-89. doi: 10.1159/000522604. Epub 2022 Feb 23. Visc Med. 2022. PMID: 35614895 Free PMC article. Review.
-
Validation of thermal dynamics during Hyperthermic IntraPEritoneal Chemotherapy simulations using a 3D-printed phantom.Front Oncol. 2023 Feb 14;13:1102242. doi: 10.3389/fonc.2023.1102242. eCollection 2023. Front Oncol. 2023. PMID: 36865797 Free PMC article.
References
-
- Appleton TG. (1997). Donor atom preferences in complexes of platinum and palladium with amino acids and related molecules. Coord Chem Rev 166:313–59.
-
- Ash S. (2003). Chronic peritoneal dialysis catheters: overview of design, placement, and removal procedures . Semin Dial 16:323–34. - PubMed
-
- Baxter LT, Jain RK. (1989). Transport of fluid and macromolecules in tumors I. Role of interstitial pressure and convection. Microvasc Res 37:77–104. - PubMed
-
- Baxter LT, Jain RK. (1990). Transport of fluid and macromolecules in tumors. II. Role of heterogeneous perfusion and lymphatics. Microvasc Res 40:246–63. - PubMed
-
- Bergs J, Haveman J, Ten Cate R, et al. (2007). Effect of 41∘c and 43∘c on cisplatin radiosensitization in two human carcinoma cell lines with different sensitivities for cisplatin. Oncol Rep 18:219–26. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
