The human papillomavirus oncoproteins: a review of the host pathways targeted on the road to transformation
- PMID: 33427604
- PMCID: PMC8148304
- DOI: 10.1099/jgv.0.001540
The human papillomavirus oncoproteins: a review of the host pathways targeted on the road to transformation
Abstract
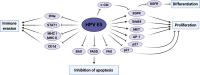
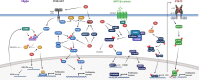
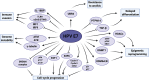
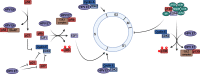
Persistent infection with high-risk human papillomaviruses (HR-HPVs) is the causal factor in over 99 % of cervical cancer cases, and a significant proportion of oropharyngeal and anogenital cancers. The key drivers of HPV-mediated transformation are the oncoproteins E5, E6 and E7. Together, they act to prolong cell-cycle progression, delay differentiation and inhibit apoptosis in the host keratinocyte cell in order to generate an environment permissive for viral replication. The oncoproteins also have key roles in mediating evasion of the host immune response, enabling infection to persist. Moreover, prolonged infection within the cellular environment established by the HR-HPV oncoproteins can lead to the acquisition of host genetic mutations, eventually culminating in transformation to malignancy. In this review, we outline the many ways in which the HR-HPV oncoproteins manipulate the host cellular environment, focusing on how these activities can contribute to carcinogenesis.
Keywords: HPV; cancer; keratinocyte; oncoprotein; signalling.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures


References
-
- PaVE The papillomavirus episteme. Available from: pave.niaid.nih.gov
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

