Obesity diminishes response to PD-1-based immunotherapies in renal cancer
- PMID: 33427691
- PMCID: PMC7757487
- DOI: 10.1136/jitc-2020-000725
Obesity diminishes response to PD-1-based immunotherapies in renal cancer
Abstract
Background: Obesity is a major risk factor for renal cancer, yet our understanding of its effects on antitumor immunity and immunotherapy outcomes remains incomplete. Deciphering these associations is critical, given the growing clinical use of immune checkpoint inhibitors for metastatic disease and mounting evidence for an obesity paradox in the context of cancer immunotherapies, wherein obese patients with cancer have improved outcomes.
Methods: We investigated associations between host obesity and anti-programmed cell death (PD-1)-based outcomes in both renal cell carcinoma (RCC) subjects and orthotopic murine renal tumors. Overall survival (OS) and progression-free survival (PFS) were determined for advanced RCC subjects receiving standard of care anti-PD-1 who had ≥6 months of follow-up from treatment initiation (n=73). Renal tumor tissues were collected from treatment-naive subjects categorized as obese (body mass index, 'BMI' ≥30 kg/m2) or non-obese (BMI <30 kg/m2) undergoing partial or full nephrectomy (n=19) then used to evaluate the frequency and phenotype of intratumoral CD8+ T cells, including PD-1 status, by flow cytometry. In mice, antitumor immunity and excised renal tumor weights were evaluated ±administration of a combinatorial anti-PD-1 therapy. For a subset of murine renal tumors, immunophenotyping was performed by flow cytometry and immunogenetic profiles were evaluated via nanoString.
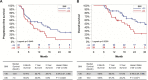
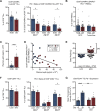
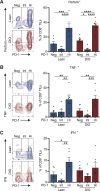
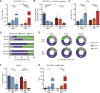
Results: With obesity, RCC patients receiving anti-PD-1 administration exhibited shorter PFS (p=0.0448) and OS (p=0.0288). Treatment-naive renal cancer subjects had decreased frequencies of tumor-infiltrating PD-1highCD8+ T cells, a finding recapitulated in our murine model. Following anti-PD-1-based immunotherapy, both lean and obese mice possessed distinct populations of treatment responders versus non-responders; however, obesity reduced the frequency of treatment responders (73% lean vs 44% obese). Tumors from lean and obese treatment responders displayed similar immunogenetic profiles, robust infiltration by PD-1int interferon (IFN)γ+CD8+ T cells and reduced myeloid-derived suppressor cells (MDSC), yielding favorable CD44+CD8+ T cell to MDSC ratios. Neutralizing interleukin (IL)-1β in obese mice improved treatment response rates to 58% and reduced MDSC accumulation in tumors.
Conclusions: We find that obesity is associated with diminished efficacy of anti-PD-1-based therapies in renal cancer, due in part to increased inflammatory IL-1β levels, highlighting the need for continued study of this critical issue.
Keywords: CD8-positive T-lymphocytes; immunotherapy; kidney neoplasms; lymphocytes; programmed cell death 1 receptor; tumor-infiltrating.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: CBR is currently employed by Immunomic Therapeutics. RCA serves as an advisory board member for Clovis, Tesaro and LEAP therapeutics. EY receives research funding from Eli Lilly and serves as an advisory board member for Bayer and AstraZeneca.
Figures


References
Publication types
MeSH terms
Grants and funding
- T32 CA183926/CA/NCI NIH HHS/United States
- P30 CA086862/CA/NCI NIH HHS/United States
- T32 HL105349/HL/NHLBI NIH HHS/United States
- T32 DK062710/DK/NIDDK NIH HHS/United States
- R25 CA047888/CA/NCI NIH HHS/United States
- T32 CA047888/CA/NCI NIH HHS/United States
- R01 CA181088/CA/NCI NIH HHS/United States
- T32 AI007051/AI/NIAID NIH HHS/United States
- S10 OD021697/OD/NIH HHS/United States
- P30 DK079626/DK/NIDDK NIH HHS/United States
- P30 CA013148/CA/NCI NIH HHS/United States
- P30 DK056336/DK/NIDDK NIH HHS/United States
- T32 GM008111/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
