Cerebrospinal fluid outflow: a review of the historical and contemporary evidence for arachnoid villi, perineural routes, and dural lymphatics
- PMID: 33427948
- PMCID: PMC8004496
- DOI: 10.1007/s00018-020-03706-5
Cerebrospinal fluid outflow: a review of the historical and contemporary evidence for arachnoid villi, perineural routes, and dural lymphatics
Abstract
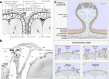
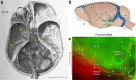
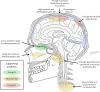
Cerebrospinal fluid (CSF) is produced by the choroid plexuses within the ventricles of the brain and circulates through the subarachnoid space of the skull and spinal column to provide buoyancy to and maintain fluid homeostasis of the brain and spinal cord. The question of how CSF drains from the subarachnoid space has long puzzled scientists and clinicians. For many decades, it was believed that arachnoid villi or granulations, outcroppings of arachnoid tissue that project into the dural venous sinuses, served as the major outflow route. However, this concept has been increasingly challenged in recent years, as physiological and imaging evidence from several species has accumulated showing that tracers injected into the CSF can instead be found within lymphatic vessels draining from the cranium and spine. With the recent high-profile rediscovery of meningeal lymphatic vessels located in the dura mater, another debate has emerged regarding the exact anatomical pathway(s) for CSF to reach the lymphatic system, with one side favoring direct efflux to the dural lymphatic vessels within the skull and spinal column and another side advocating for pathways along exiting cranial and spinal nerves. In this review, a summary of the historical and contemporary evidence for the different outflow pathways will be presented, allowing the reader to gain further perspective on the recent advances in the field. An improved understanding of this fundamental physiological process may lead to novel therapeutic approaches for a wide range of neurological conditions, including hydrocephalus, neurodegeneration and multiple sclerosis.
Keywords: CSF; Clearance; Cranial nerves; Cribriform plate; Lymphatic vessels; Meningeal.
Conflict of interest statement
The author declares that he has no conflict of interest.
Figures


References
-
- Key A, Retzius G. Studien in der Anatomie des Nervensystems und des Bindegewebes. Stockholm: Samson & Waller; 1875.
-
- Davson H, Segal MB. Physiology of the CSF and blood–brain barriers. Boca Raton: CRC Press; 1996.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

