CCN2 (Cellular Communication Network factor 2) in the bone marrow microenvironment, normal and malignant hematopoiesis
- PMID: 33428075
- PMCID: PMC7798015
- DOI: 10.1007/s12079-020-00602-2
CCN2 (Cellular Communication Network factor 2) in the bone marrow microenvironment, normal and malignant hematopoiesis
Abstract
CCN2, formerly termed Connective Tissue Growth Factor, is a protein belonging to the Cellular Communication Network (CCN)-family of secreted extracellular matrix-associated proteins. As a matricellular protein it is mainly considered to be active as a modifier of signaling activity of several different signaling pathways and as an orchestrator of their cross-talk. Furthermore, CCN2 and its fragments have been implicated in the regulation of a multitude of biological processes, including cell proliferation, differentiation, adhesion, migration, cell survival, apoptosis and the production of extracellular matrix products, as well as in more complex processes such as embryonic development, angiogenesis, chondrogenesis, osteogenesis, fibrosis, mechanotransduction and inflammation. Its function is complex and context dependent, depending on cell type, state of differentiation and microenvironmental context. CCN2 plays a role in many diseases, especially those associated with fibrosis, but has also been implicated in many different forms of cancer. In the bone marrow (BM), CCN2 is highly expressed in mesenchymal stem/stromal cells (MSCs). CCN2 is important for MSC function, supporting its proliferation, migration and differentiation. In addition, stromal CCN2 supports the maintenance and longtime survival of hematopoietic stem cells, and in the presence of interleukin 7, stimulates the differentiation of pro-B lymphocytes into pre-B lymphocytes. Overexpression of CCN2 is seen in the majority of B-acute lymphoblastic leukemias, especially in certain cytogenetic subgroups associated with poor outcome. In acute myeloid leukemia, CCN2 expression is increased in MSCs, which has been associated with leukemic engraftment in vivo. In this review, the complex function of CCN2 in the BM microenvironment and in normal as well as malignant hematopoiesis is discussed. In addition, an overview is given of data on the remaining CCN family members regarding normal and malignant hematopoiesis, having many similarities and some differences in their function.
Keywords: Bone marrow; CCN2; CTGF; Connective tissue growth factor; Hematopoiesis; Leukemogenesis.
Conflict of interest statement
The authors report no conflict of interest.
Figures



 , is present in different bone marrow (BM) mesenchymal cells, including endothelial cells, osteoblasts, adipocytes and fibroblasts, with highest levels (
, is present in different bone marrow (BM) mesenchymal cells, including endothelial cells, osteoblasts, adipocytes and fibroblasts, with highest levels ( ) reported in mesenchymal stem/stromal cell (MSC) and CXCL12-abundant reticular (CAR) cells. CCN2 exerts different actions in the BM. a In the presence of interleukin 7 (IL-7), CCN2 promotes pro-B cell to pre-B cell differentiation. b CCN2 produced by MSCs affects the (long-term) qualities of hematopoietic stem cells (HSCs). HSCs, in turn, upregulate CCN2 expression by MSCs. c CCN2 enhances the differentiation of MSCs into endothelial cells, osteoblast and fibroblasts, but has an inhibitory effect on the differentiation of MSCs into adipocytes. d CCN2 might induce the production of the ECM proteins collagen type I and type III, fibronectin, decorin, TGFβ-2 and lysyl oxidase by fibroblast. e CCN2 binds to fibronectin, perlecan and decorin, known constituents of the BM extracellular matrix. The effects hereof in the BM are yet unknown.
) reported in mesenchymal stem/stromal cell (MSC) and CXCL12-abundant reticular (CAR) cells. CCN2 exerts different actions in the BM. a In the presence of interleukin 7 (IL-7), CCN2 promotes pro-B cell to pre-B cell differentiation. b CCN2 produced by MSCs affects the (long-term) qualities of hematopoietic stem cells (HSCs). HSCs, in turn, upregulate CCN2 expression by MSCs. c CCN2 enhances the differentiation of MSCs into endothelial cells, osteoblast and fibroblasts, but has an inhibitory effect on the differentiation of MSCs into adipocytes. d CCN2 might induce the production of the ECM proteins collagen type I and type III, fibronectin, decorin, TGFβ-2 and lysyl oxidase by fibroblast. e CCN2 binds to fibronectin, perlecan and decorin, known constituents of the BM extracellular matrix. The effects hereof in the BM are yet unknown.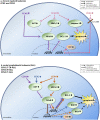
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

