Leadless pacemaker implant with concomitant atrioventricular node ablation: Experience with the Micra transcatheter pacemaker
- PMID: 33428248
- PMCID: PMC7986103
- DOI: 10.1111/jce.14881
Leadless pacemaker implant with concomitant atrioventricular node ablation: Experience with the Micra transcatheter pacemaker
Abstract
Background: The feasibility and outcomes of concomitant atrioventricular node ablation (AVNA) and leadless pacemaker implant are not well studied. We report outcomes in patients undergoing Micra implant with concomitant AVNA.
Methods: Patients undergoing AVNA at the time of Micra implant from the Micra Transcatheter Pacing (IDE) Study, Continued Access (CA) study, and Post-Approval Registry (PAR) were included in the analysis and compared to Micra patients without AVNA. Baseline characteristics, acute and follow-up outcomes, and electrical performance were compared between patients with and without AVNA during the follow-up period.
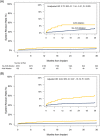
Results: A total of 192 patients (mean age 77.4 ± 8.9 years, 72% female) underwent AVNA at the time of Micra implant and were followed for 20.4 ± 15.6 months. AVNA patients were older, more frequently female, and tended to have more co-morbid conditions compared with non-AVNA patients (N = 2616). Implant was successful in 191 of 192 patients (99.5%). The mean pacing threshold at implant was 0.58 ± 0.35 V and remained stable during follow-up. Major complications within 30 days occurred more frequently in AVNA patients than non-AVNA patients (7.3% vs. 2.0%, p < .001). The risk of major complications through 36-months was higher in AVNA patients (hazard ratio: 3.81, 95% confidence interval: 2.33-6.23, p < .001). Intermittent loss of capture occurred in three AVNA patients (1.6%), all were within 30 days of implant and required system revision. There were no device macrodislodgements or unexpected device malfunctions.
Conclusion: Concomitant AVN ablation and leadless pacemaker implant is feasible. Pacing thresholds are stable over time. However, patient comorbidities and the risk of major complications are higher in patients undergoing AVNA.
Trial registration: ClinicalTrials.gov NCT02536118 NCT02488681 NCT02004873.
Keywords: AV node ablation; Micra; leadless pacemaker.
© 2021 The Authors. Journal of Cardiovascular Electrophysiology Published by Wiley Periodicals LLC.
Figures



References
-
- Lee SH, Chen SA, Tai CT, et al. Comparisons of quality of life and cardiac performance after complete atrioventricular junction ablation and atrioventricular junction modification in patients with medically refractory atrial fibrillation. J Am Coll Cardiol. 1998;31(3):637‐644. - PubMed
-
- Kay GN, Ellenbogen KA, Giudici M, et al. The Ablate and Pace Trial: a prospective study of catheter ablation of the AV conduction system and permanent pacemaker implantation for treatment of atrial fibrillation. APT Investigators. J Interv Card Electrophysiol. 1998;2(2):121‐135. - PubMed
-
- Hoffmayer KS, Scheinman M. Current role of atrioventricular junction (AVJ) ablation. Pacing Clin Electrophysiol. 2013;36(2):257‐265. - PubMed
-
- Kuck KH, Brugada J, Fürnkranz A, et al. Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Engl J Med. 2016;374(23):2235‐2245. - PubMed
-
- Cantillon DJ, Exner DV, Badie N, et al. Complications and health care costs associated with transvenous cardiac pacemakers in a nationwide assessment. JACC Clin Electrophysiol. 2017;3(11):1296‐1305. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

