Total Phenolic Fraction (TPF) from Extra Virgin Olive Oil: Induction of apoptotic-like cell death in Leishmania spp. promastigotes and in vivo potential of therapeutic immunomodulation
- PMID: 33428610
- PMCID: PMC7799795
- DOI: 10.1371/journal.pntd.0008968
Total Phenolic Fraction (TPF) from Extra Virgin Olive Oil: Induction of apoptotic-like cell death in Leishmania spp. promastigotes and in vivo potential of therapeutic immunomodulation
Abstract
Background: Leishmaniasis is a serious multifactorial parasitic disease with limited treatment options. Current chemotherapy is mainly consisted of drugs with serious drawbacks such as toxicity, variable efficacy and resistance. Alternative bioactive phytocompounds may provide a promising source for discovering new anti-leishmanial drugs. Extra Virgin Olive Oil (EVOO), a key-product in the Mediterranean diet, is rich in phenols which are associated with anti-inflammatory, anti-cancer and anti-microbial effects. In this study, we investigate the anti-leishmanial effect of Total Phenolic Fraction (TPF) derived from EVOO in both in vitro and in vivo systems by investigating the contributing mechanism of action.
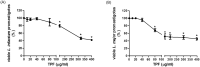
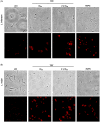
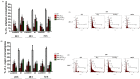
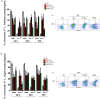
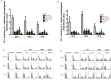
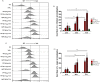
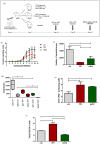
Methodology/principal findings: We tested the ability of TPF to cause apoptotic-like programmed cell death in L. infantum and L. major exponential-phase promastigotes by evaluating several apoptotic indices, such as reduction of proliferation rate, sub-G0/G1 phase cell cycle arrest, phosphatidylserine externalization, mitochondrial transmembrane potential disruption and increased ROS production, by using flow cytometry and microscopy techniques. Moreover, we assessed the therapeutic effect of TPF in L. major-infected BALB/c mice by determining skin lesions, parasite burden in popliteal lymph nodes, Leishmania-specific antibodies and biomarkers of tissue site cellular immune response, five weeks post-treatment termination. Our results show that TPF triggers cell-cycle arrest and apoptotic-like changes in Leishmania spp. promastigotes. Moreover, TPF treatment induces significant reduction of parasite burden in draining lymph nodes together with an antibody profile indicative of the polarization of Th1/Th2 immune balance towards the protective Th1-type response, characterized by the presence of IFN-γ-producing CD4+ T-cells and increased Tbx21/GATA-3 gene expression ratio in splenocytes.
Conclusions/significance: TPF exhibits chemotherapeutic anti-leishmanial activity by inducing programmed cell death on cell-free promastigotes and immunomodulatory properties that induce in vivo T cell-mediated responses towards the protective Th1 response in experimental cutaneous leishmaniasis. These findings enable deeper understanding of TPF's dual mode of action that encourages further studies.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
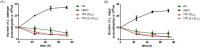
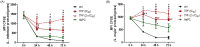
Similar articles
-
Direct In Vitro Comparison of the Anti-Leishmanial Activity of Different Olive Oil Total Polyphenolic Fractions and Assessment of Their Combined Effects with Miltefosine.Molecules. 2022 Sep 21;27(19):6176. doi: 10.3390/molecules27196176. Molecules. 2022. PMID: 36234713 Free PMC article.
-
Evaluation of total phenolic fraction derived from extra virgin olive oil for its antileishmanial activity.Phytomedicine. 2018 Aug 1;47:143-150. doi: 10.1016/j.phymed.2018.04.030. Epub 2018 May 10. Phytomedicine. 2018. PMID: 30166099
-
The leishmanicidal activity of oleuropein is selectively regulated through inflammation- and oxidative stress-related genes.Parasit Vectors. 2016 Aug 9;9(1):441. doi: 10.1186/s13071-016-1701-4. Parasit Vectors. 2016. PMID: 27501956 Free PMC article.
-
Anti-leishmania effector functions of CD4+ Th1 cells and early events instructing Th2 cell development and susceptibility to Leishmania major in BALB/c mice.Adv Exp Med Biol. 1998;452:53-60. doi: 10.1007/978-1-4615-5355-7_7. Adv Exp Med Biol. 1998. PMID: 9889959 Review.
-
[Immunopathology of American tegumentary leishmaniasis].Acta Cient Venez. 1998;49(1):42-56. Acta Cient Venez. 1998. PMID: 10205916 Review. Spanish.
Cited by
-
Leishmania Protein Kinases: Important Regulators of the Parasite Life Cycle and Molecular Targets for Treating Leishmaniasis.Microorganisms. 2021 Mar 27;9(4):691. doi: 10.3390/microorganisms9040691. Microorganisms. 2021. PMID: 33801655 Free PMC article. Review.
-
The Role of Metacaspases and Other Proteins Involved in the Apoptosis of Leishmania: Review Article.Iran J Parasitol. 2025 Jan-Mar;20(1):1-12. doi: 10.18502/ijpa.v20i1.18100. Iran J Parasitol. 2025. PMID: 40206367 Free PMC article. Review.
-
Leishmanicidal Activity of Guanidine Derivatives against Leishmania infantum.Trop Med Infect Dis. 2023 Feb 25;8(3):141. doi: 10.3390/tropicalmed8030141. Trop Med Infect Dis. 2023. PMID: 36977142 Free PMC article.
-
Direct In Vitro Comparison of the Anti-Leishmanial Activity of Different Olive Oil Total Polyphenolic Fractions and Assessment of Their Combined Effects with Miltefosine.Molecules. 2022 Sep 21;27(19):6176. doi: 10.3390/molecules27196176. Molecules. 2022. PMID: 36234713 Free PMC article.
-
Anti-Leishmania major activity of Calotropis procera extract by increasing ROS production and upregulating TNF-α, IFN-γ and iNOS mRNA expression under in vitro conditions.Trop Med Health. 2024 Feb 1;52(1):16. doi: 10.1186/s41182-024-00578-4. Trop Med Health. 2024. PMID: 38303082 Free PMC article.
References
-
- Matos APS, Vicosa AL, Re MI, Ricci E, Holandino C. A review of current treatments strategies based on paromomycin for leishmaniasis. J Drug Deliv Sci Tec. 2020;57. Artn 10166410.1016/J.Jddst.2020.101664.
-
- Hailu A, Musa A, Wasunna M, Balasegaram M, Yifru S, Mengistu G, et al. Geographical Variation in the Response of Visceral Leishmaniasis to Paromomycin in East Africa: A Multicentre, Open-Label, Randomized Trial. PLoS neglected tropical diseases. 2010;4(10). ARTN e709 10.1371/journal.pntd.0000709 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

