Host-specific functional compartmentalization within the oligopeptide transporter during the Borrelia burgdorferi enzootic cycle
- PMID: 33428666
- PMCID: PMC7822543
- DOI: 10.1371/journal.ppat.1009180
Host-specific functional compartmentalization within the oligopeptide transporter during the Borrelia burgdorferi enzootic cycle
Abstract
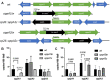
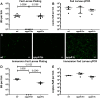
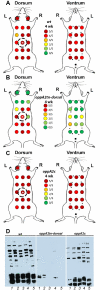
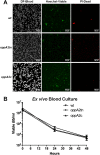
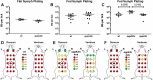
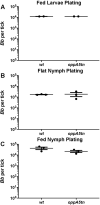
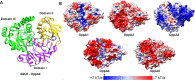
Borrelia burgdorferi must acquire all of its amino acids (AAs) from its arthropod vector and vertebrate host. Previously, we determined that peptide uptake via the oligopeptide (Opp) ABC transporter is essential for spirochete viability in vitro and during infection. Our prior study also suggested that B. burgdorferi employs temporal regulation in concert with structural variation of oligopeptide-binding proteins (OppAs) to meet its AA requirements in each biological niche. Herein, we evaluated the contributions to the B. burgdorferi enzootic cycle of three of the spirochete's five OppAs (OppA1, OppA2, and OppA5). An oppA1 transposon (tn) mutant lysed in the hyperosmolar environment of the feeding tick, suggesting that OppA1 imports amino acids required for osmoprotection. The oppA2tn mutant displayed a profound defect in hematogenous dissemination in mice, yet persisted within skin while inducing only a minimal antibody response. These results, along with slightly decreased growth of the oppA2tn mutant within DMCs, suggest that OppA2 serves a minor nutritive role, while its dissemination defect points to an as yet uncharacterized signaling function. Previously, we identified a role for OppA5 in spirochete persistence within the mammalian host. We now show that the oppA5tn mutant displayed no defect during the tick phase of the cycle and could be tick-transmitted to naïve mice. Instead of working in tandem, however, OppA2 and OppA5 appear to function in a hierarchical manner; the ability of OppA5 to promote persistence relies upon the ability of OppA2 to facilitate dissemination. Structural homology models demonstrated variations within the binding pockets of OppA1, 2, and 5 indicative of different peptide repertoires. Rather than being redundant, B. burgdorferi's multiplicity of Opp binding proteins enables host-specific functional compartmentalization during the spirochete lifecycle.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Peptide Uptake Is Essential for Borrelia burgdorferi Viability and Involves Structural and Regulatory Complexity of its Oligopeptide Transporter.mBio. 2017 Dec 19;8(6):e02047-17. doi: 10.1128/mBio.02047-17. mBio. 2017. PMID: 29259089 Free PMC article.
-
Borrelia burgdorferi requires glycerol for maximum fitness during the tick phase of the enzootic cycle.PLoS Pathog. 2011 Jul;7(7):e1002102. doi: 10.1371/journal.ppat.1002102. Epub 2011 Jul 7. PLoS Pathog. 2011. PMID: 21750672 Free PMC article.
-
Regulatory protein BBD18 of the lyme disease spirochete: essential role during tick acquisition?mBio. 2014 Apr 1;5(2):e01017-14. doi: 10.1128/mBio.01017-14. mBio. 2014. PMID: 24692636 Free PMC article.
-
Borrelia burgdorferi protein interactions critical for microbial persistence in mammals.Cell Microbiol. 2019 Feb;21(2):e12885. doi: 10.1111/cmi.12885. Epub 2018 Jul 8. Cell Microbiol. 2019. PMID: 29934966 Free PMC article. Review.
-
Interaction of the Lyme disease spirochete with its tick vector.Cell Microbiol. 2016 Jul;18(7):919-27. doi: 10.1111/cmi.12609. Epub 2016 May 24. Cell Microbiol. 2016. PMID: 27147446 Free PMC article. Review.
Cited by
-
Dissection of amino acid acquisition pathways in Borrelia burgdorferi uncovers unique physiological responses.bioRxiv [Preprint]. 2025 Mar 15:2025.03.14.643351. doi: 10.1101/2025.03.14.643351. bioRxiv. 2025. PMID: 40161780 Free PMC article. Preprint.
-
Antibacterial Ingredients and Modes of the Methanol-Phase Extract from the Fruit of Amomum villosum Lour.Plants (Basel). 2024 Mar 14;13(6):834. doi: 10.3390/plants13060834. Plants (Basel). 2024. PMID: 38592864 Free PMC article.
-
PlzA is a bifunctional c-di-GMP biosensor that promotes tick and mammalian host-adaptation of Borrelia burgdorferi.PLoS Pathog. 2021 Jul 15;17(7):e1009725. doi: 10.1371/journal.ppat.1009725. eCollection 2021 Jul. PLoS Pathog. 2021. PMID: 34265024 Free PMC article.
-
The arginine deaminase system plays distinct roles in Borrelia burgdorferi and Borrelia hermsii.PLoS Pathog. 2022 Mar 14;18(3):e1010370. doi: 10.1371/journal.ppat.1010370. eCollection 2022 Mar. PLoS Pathog. 2022. PMID: 35286343 Free PMC article.
-
Pathogenicity and virulence of Borrelia burgdorferi.Virulence. 2023 Dec;14(1):2265015. doi: 10.1080/21505594.2023.2265015. Epub 2023 Oct 9. Virulence. 2023. PMID: 37814488 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

