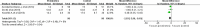The efficacy and safety of prokinetics in critically ill adults receiving gastric feeding tubes: A systematic review and meta-analysis
- PMID: 33428672
- PMCID: PMC7799841
- DOI: 10.1371/journal.pone.0245317
The efficacy and safety of prokinetics in critically ill adults receiving gastric feeding tubes: A systematic review and meta-analysis
Abstract
Background: Intolerance to gastric feeding tubes is common among critically ill adults and may increase morbidity. Administration of prokinetics in the ICU is common. However, the efficacy and safety of prokinetics are unclear in critically ill adults with gastric feeding tubes. We conducted a systematic review to determine the efficacy and safety of prokinetics for improving gastric feeding tube tolerance in critically ill adults.
Methods: Randomized controlled trials (RCTs) were identified by systematically searching the Medline, Cochrane and Embase databases. Two independent reviewers extracted the relevant data and assessed the quality of the studies. We calculated pooled relative risks (RRs) for dichotomous outcomes and the mean differences (MDs) for continuous outcomes with the corresponding 95% confidence intervals (CIs). We assessed the risk of bias using the Cochrane risk-of-bias tool and used the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) methodology to rate the quality of the evidence.
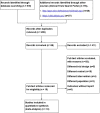
Results: Fifteen RCTs met the inclusion criteria. A total of 10 RCTs involving 846 participants were eligible for the quantitative analysis. Most studies (10 of 13, 76.92%) showed that prokinetics had beneficial effects on feeding intolerance in critically ill adults. In critically ill adults receiving gastric feeding, prokinetic agents may reduce the ICU length of stay (MD -2.03, 95% CI -3.96, -0.10; P = 0.04; low certainty) and the hospital length of stay (MD -3.21, 95% CI -5.35, -1.06; P = 0.003; low certainty). However, prokinetics failed to improve the outcomes of reported adverse events and all-cause mortality.
Conclusion: As a class of drugs, prokinetics may improve tolerance to gastric feeding to some extent in critically ill adults. However, the certainty of the evidence suggesting that prokinetics reduce the ICU or hospital length of stay is low. Prokinetics did not significantly decrease the risks of reported adverse events or all-cause mortality among critically ill adults.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Canadian clinical practice guidelines for nutrition support in mechanically ventilated, critically ill adult patients.JPEN J Parenter Enteral Nutr. 2003 Sep-Oct;27(5):355-73. doi: 10.1177/0148607103027005355. JPEN J Parenter Enteral Nutr. 2003. PMID: 12971736
-
The Efficacy and Safety of In-Intensive Care Unit Leg-Cycle Ergometry in Critically Ill Adults. A Systematic Review and Meta-analysis.Ann Am Thorac Soc. 2020 Oct;17(10):1289-1307. doi: 10.1513/AnnalsATS.202001-059OC. Ann Am Thorac Soc. 2020. PMID: 32628501
-
Effects of high-fat, low-carbohydrate enteral nutrition in critically ill patients: A systematic review with meta-analysis.Clin Nutr. 2024 Oct;43(10):2399-2406. doi: 10.1016/j.clnu.2024.09.023. Epub 2024 Sep 12. Clin Nutr. 2024. PMID: 39288649
-
Leg Cycle Ergometry in Critically Ill Patients - An Updated Systematic Review and Meta-Analysis.NEJM Evid. 2024 Dec;3(12):EVIDoa2400194. doi: 10.1056/EVIDoa2400194. Epub 2024 Oct 9. NEJM Evid. 2024. PMID: 39382351
-
Current evidence on ω-3 fatty acids in enteral nutrition in the critically ill: A systematic review and meta-analysis.Nutrition. 2019 Mar;59:56-68. doi: 10.1016/j.nut.2018.07.013. Epub 2018 Jul 31. Nutrition. 2019. PMID: 30419501
Cited by
-
Use of prokinetic agents in hospitalised adult patients: Protocol for a scoping review.Acta Anaesthesiol Scand. 2022 Sep;66(8):1024-1026. doi: 10.1111/aas.14099. Epub 2022 Jun 22. Acta Anaesthesiol Scand. 2022. PMID: 35675417 Free PMC article.
-
Major Publications in the Critical Care Pharmacotherapy Literature: 2021.Crit Care Explor. 2022 Dec 14;4(12):e0823. doi: 10.1097/CCE.0000000000000823. eCollection 2022 Dec. Crit Care Explor. 2022. PMID: 36567788 Free PMC article. Review.
-
Serial Measurements of Refractive Index, Glucose and Protein to Assess Gastric Liquid Nutrient Transport-A Proof-of-Principal Study.Front Nutr. 2022 Feb 3;8:742656. doi: 10.3389/fnut.2021.742656. eCollection 2021. Front Nutr. 2022. PMID: 35187015 Free PMC article.
-
Efficacy and safety of interleukin-6 receptor antagonists in adult patients admitted to intensive care unit with COVID-19: A systematic review and meta-analysis of randomized controlled trials.Prev Med Rep. 2023 Aug;34:102276. doi: 10.1016/j.pmedr.2023.102276. Epub 2023 Jun 7. Prev Med Rep. 2023. PMID: 37309358 Free PMC article. Review.
-
Gastrointestinal dysfunction during enteral nutrition delivery in intensive care unit (ICU) patients: Risk factors, natural history, and clinical implications. A post-hoc analysis of The Augmented versus Routine approach to Giving Energy Trial (TARGET).Am J Clin Nutr. 2022 Aug 4;116(2):589-598. doi: 10.1093/ajcn/nqac113. Am J Clin Nutr. 2022. PMID: 35472097 Free PMC article. Clinical Trial.
References
-
- McClave SA, Taylor BE, Martindale RG, Warren MM, Johnson DR, Braunschweig C, et al. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J Parenter Enteral Nutr. 2016;40(2):159–211. Epub 2016/01/17. 10.1177/0148607115621863 . - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous