Recent advances in nanomaterials for therapy and diagnosis for atherosclerosis
- PMID: 33428994
- PMCID: PMC7981266
- DOI: 10.1016/j.addr.2021.01.005
Recent advances in nanomaterials for therapy and diagnosis for atherosclerosis
Abstract
Atherosclerosis is a chronic inflammatory disease driven by lipid accumulation in arteries, leading to narrowing and thrombosis. It affects the heart, brain, and peripheral vessels and is the leading cause of mortality in the United States. Researchers have strived to design nanomaterials of various functions, ranging from non-invasive imaging contrast agents, targeted therapeutic delivery systems to multifunctional nanoagents able to target, diagnose, and treat atherosclerosis. Therefore, this review aims to summarize recent progress (2017-now) in the development of nanomaterials and their applications to improve atherosclerosis diagnosis and therapy during the preclinical and clinical stages of the disease.
Keywords: Atherosclerosis; Clinical use; Imaging contrast agents; Nanomaterials; Theranostic agents; Therapeutic delivery system.
Copyright © 2021 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare no conflict of interest in the publication of this work.
Figures

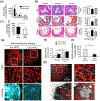
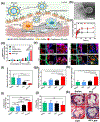

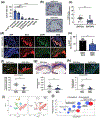

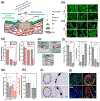





References
-
- W.H. Organization, Cardiovascular diseases (CVDs) fact sheet, World Health Organization, (2017).
-
- d SJ, Giri J, Kobayashi T, Stent-based revascularization for complex lesions in PAD, J Cardiovasc Surg, 58 (2017) 715–721. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

