Nanocarrier vaccines for SARS-CoV-2
- PMID: 33428995
- PMCID: PMC7794055
- DOI: 10.1016/j.addr.2021.01.002
Nanocarrier vaccines for SARS-CoV-2
Abstract
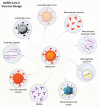
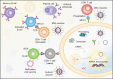
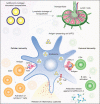
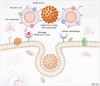
The SARS-CoV-2 global pandemic has seen rapid spread, disease morbidities and death associated with substantive social, economic and societal impacts. Treatments rely on re-purposed antivirals and immune modulatory agents focusing on attenuating the acute respiratory distress syndrome. No curative therapies exist. Vaccines remain the best hope for disease control and the principal global effort to end the pandemic. Herein, we summarize those developments with a focus on the role played by nanocarrier delivery.
Keywords: COVID-19 vaccine; Nanovaccine; SARS-CoV-2; mRNA vaccine.
Crown Copyright © 2021. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare no conflicts of interest.
Figures



References
-
- World Health Organization . 2020. Naming the coronavirus disease (COVID-19) and the virus that causes it.https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technica...
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

