The innate immune system in diabetic retinopathy
- PMID: 33429059
- PMCID: PMC8263813
- DOI: 10.1016/j.preteyeres.2021.100940
The innate immune system in diabetic retinopathy
Abstract
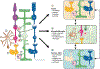
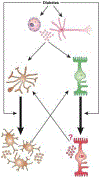
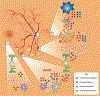
The prevalence of diabetes has been rising steadily in the past half-century, along with the burden of its associated complications, including diabetic retinopathy (DR). DR is currently the most common cause of vision loss in working-age adults in the United States. Historically, DR has been diagnosed and classified clinically based on what is visible by fundoscopy; that is vasculature alterations. However, recent technological advances have confirmed pathology of the neuroretina prior to any detectable vascular changes. These, coupled with molecular studies, and the positive impact of anti-inflammatory therapeutics in DR patients have highlighted the central involvement of the innate immune system. Reminiscent of the systemic impact of diabetes, immune dysregulation has become increasingly identified as a key element of the pathophysiology of DR by interfering with normal homeostatic systems. This review uses the growing body of literature across various model systems to demonstrate the clear involvement of all three pillars of the immune system: immune-competent cells, mediators, and the complement system. It also demonstrates how the relative contribution of each of these requires more extensive analysis, including in human tissues over the continuum of disease progression. Finally, although this review demonstrates how the complex interactions of the immune system pose many more questions than answers, the intimately connected nature of the three pillars of the immune system may also point to possible new targets to reverse or even halt reverse retinopathy.
Keywords: Complement system; Diabetic retinopathy; Innate immunity; Microglia and macroglia; Neurodegeneration; Neurovascular unit.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors have declared that no conflict of interest exists.
Figures

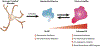



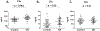
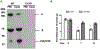
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

