Recombinant antibody against Trypanosoma cruzi from patients with chronic Chagas heart disease recognizes mammalian nervous system
- PMID: 33429173
- PMCID: PMC7809174
- DOI: 10.1016/j.ebiom.2020.103206
Recombinant antibody against Trypanosoma cruzi from patients with chronic Chagas heart disease recognizes mammalian nervous system
Abstract
Background: To deeply understand the role of antibodies in the context of Trypanosoma cruzi infection, we decided to characterize A2R1, a parasite antibody selected from single-chain variable fragment (scFv) phage display libraries constructed from B cells of chronic Chagas heart disease patients.
Methods: Immunoblot, ELISA, cytometry, immunofluorescence and immunohistochemical assays were used to characterize A2R1 reactivity. To identify the antibody target, we performed an immunoprecipitation and two-dimensional electrophoresis coupled to mass spectrometry and confirmed A2R1 specific interaction by producing the antigen in different expression systems. Based on these data, we carried out a comparative in silico analysis of the protein target´s orthologues, focusing mainly on post-translational modifications.
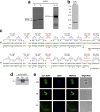
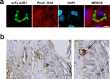
Findings: A2R1 recognizes a parasite protein of ~50 kDa present in all life cycle stages of T. cruzi, as well as in other members of the kinetoplastid family, showing a defined immunofluorescence labeling pattern consistent with the cytoskeleton. A2R1 binds to tubulin, but this interaction relies on its post-translational modifications. Interestingly, this antibody also targets mammalian tubulin only present in brain, staining in and around cell bodies of the human peripheral and central nervous system.
Interpretation: Our findings demonstrate for the first time the existence of a human antibody against T. cruzi tubulin capable of cross-reacting with a human neural protein. This work re-emphasizes the role of molecular mimicry between host and parasitic antigens in the development of pathological manifestations of T. cruzi infection.
Keywords: Chagas disease; Digestive system; Molecular mimicry; Phage-display; Post-translational modification; Tubulin.
Copyright © 2021 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interests The authors have declared that no conflict of interest exists.
Figures






References
-
- World Health Organization (WHO) IV WHO Report on Neglected Tropical Diseases; 2017. Fourth WHO report on neglected tropical diseases: integrating neglected tropical diseases into global health and development.
-
- Bern C. Chagas’ disease. N Engl J Med. 2015;373(5):456–466. - PubMed
-
- Pérez-Molina J.A., Molina I. Chagas disease. Lancet. 2017;391:82–94. - PubMed
-
- Rassi A., Rassi A., Marin-Neto J.A. Chagas disease. Lancet. 2010;375(9723):1388–1402. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

