Prospective, double-blind, randomized, placebo-controlled phase III study evaluating efficacy and safety of octagam 10% in patients with dermatomyositis ("ProDERM Study")
- PMID: 33429735
- PMCID: PMC7793357
- DOI: 10.1097/MD.0000000000023677
Prospective, double-blind, randomized, placebo-controlled phase III study evaluating efficacy and safety of octagam 10% in patients with dermatomyositis ("ProDERM Study")
Abstract
Introduction: Dermatomyositis (DM) is an inflammatory myopathy characterized by distinct skin manifestations and muscle weakness. Intravenous immunoglobulin (IVIg) has been used off-label as adjuvant therapy in DM, but is not indicated for DM, due to lack of proven efficacy in a large randomized controlled trial. The objective of the ProDERM (Progress in DERMatomyositis) study was to evaluate the efficacy, safety and long-term tolerability of IVIg (Octagam 10%) in patients with DM in a randomized, placebo-controlled, double-blind, Phase III study.
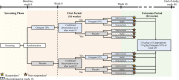
Methods: Adult patients with active DM who were continuing standard therapy at a stable dose were eligible for this study. Patients were randomized 1:1 to receive either 2 g/kg of IVIg or placebo, administered every 4 weeks until week 16 (First Period). Patients were switched to the alternate treatment if they showed clinical deterioration in the First Period. After response assessment at week 16, all patients on placebo and those without deterioration on IVIg entered the open-label Extension Period, receiving 2 g/kg IVIg every 4 weeks for 24 weeks.
Results: The primary efficacy endpoint was the proportion of responders in the IVIg vs placebo arm at week 16, where response was defined per 2016 ACR/EULAR Myositis Response Criteria of at least minimal improvement [Total Improvement Score (TIS) ≥20] and without deterioration at 2 consecutive visits up to week 16. TIS consists of composite response criteria, combining weighted improvement in 6 core set measures (CSMs), Global Disease Activity (Physician and Patient), manual muscle testing-8 (MMT-8), Health Assessment Questionnaire, extra-muscular disease activity, and muscle enzymes. Secondary endpoints included the mean change in individual CSMs, time to improvement in TIS, time to confirmed deterioration in the First Period, and the overall proportion of patients with deteriorations. Adverse events, including infusion reactions and thromboembolic events, were recorded.
Conclusions: The ProDERM study was the first to assess the long-term efficacy and safety of IVIg (Octagam 10%) in a placebo-controlled, blinded, randomized trial in DM. The study aimed to inform on the use of IVIg in the treatment of DM, and results are expected in Q3 2020.
Clinicaltrialsgov identifier: NCT02728752.
Copyright © 2021 the Author(s). Published by Wolters Kluwer Health, Inc.
Figures
References
-
- Dalakas MC. Inflammatory muscle diseases. N Engl J Med 2015;372:1734–47. - PubMed
-
- Donofrio PD, Berger A, Brannagan TH, et al. Consensus statement: the use of intravenous immunoglobulin in the treatment of neuromuscular conditions report of the AANEM ad hoc committee. Muscle Nerve 2009;40:890–900. - PubMed
-
- Elovaara I, Apostolski S, van Doorn P, et al. EFNS guidelines for the use of intravenous immunoglobulin in treatment of neurological diseases: EFNS task force on the use of intravenous immunoglobulin in treatment of neurological diseases. Eur J Neurol 2008;15:893–908. - PubMed
-
- Enk A. Guidelines on the use of high-dose intravenous immunoglobulin in dermatology. Eur J Dermatol 2009;19:90–8. - PubMed
-
- Danieli MG, Calcabrini L, Calabrese V, et al. Intravenous immunoglobulin as add on treatment with mycophenolate mofetil in severe myositis. Autoimmun Rev 2009;9:124–7. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical