N-Glycosylation in Piroplasmids: Diversity within Simplicity
- PMID: 33429911
- PMCID: PMC7826898
- DOI: 10.3390/pathogens10010050
N-Glycosylation in Piroplasmids: Diversity within Simplicity
Abstract
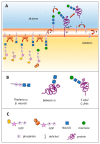
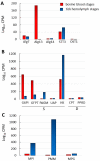
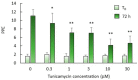
N-glycosylation has remained mostly unexplored in Piroplasmida, an order of tick-transmitted pathogens of veterinary and medical relevance. Analysis of 11 piroplasmid genomes revealed three distinct scenarios regarding N-glycosylation: Babesia sensu stricto (s.s.) species add one or two N-acetylglucosamine (NAcGlc) molecules to proteins; Theileria equi and Cytauxzoon felis add (NAcGlc)2-mannose, while B. microti and Theileria s.s. synthesize dolichol-P-P-NAcGlc and dolichol-P-P-(NAcGlc)2 without subsequent transfer to proteins. All piroplasmids possess the gene complement needed for the synthesis of the N-glycosylation substrates, dolichol-P and sugar nucleotides. The oligosaccharyl transferase of Babesia species, T. equi and C. felis, is predicted to be composed of only two subunits, STT3 and Ost1. Occurrence of short N-glycans in B. bovis merozoites was experimentally demonstrated by fluorescence microscopy using a NAcGlc-specific lectin. In vitro growth of B. bovis was significantly impaired by tunicamycin, an inhibitor of N-glycosylation, indicating a relevant role for N-glycosylation in this pathogen. Finally, genes coding for N-glycosylation enzymes and substrate biosynthesis are transcribed in B. bovis blood and tick stages, suggesting that this pathway is biologically relevant throughout the parasite life cycle. Elucidation of the role/s exerted by N-glycans will increase our understanding of these successful parasites, for which improved control measures are needed.
Keywords: Babesia; Cytauxzoon; N-glycan; Theileria; dolichol; piroplasmids; sugar nucleotides.
Conflict of interest statement
The authors declare no conflict of interest related to this work.
Figures

Similar articles
-
The Piroplasmida Babesia, Cytauxzoon, and Theileria in farm and companion animals: species compilation, molecular phylogeny, and evolutionary insights.Parasitol Res. 2022 May;121(5):1207-1245. doi: 10.1007/s00436-022-07424-8. Epub 2022 Jan 31. Parasitol Res. 2022. PMID: 35098377 Review.
-
The repertoire of serine rhomboid proteases of piroplasmids of importance to animal and human health.Int J Parasitol. 2021 May;51(6):455-462. doi: 10.1016/j.ijpara.2020.10.010. Epub 2021 Feb 19. Int J Parasitol. 2021. PMID: 33610524
-
Cysteine Proteinase C1A Paralog Profiles Correspond with Phylogenetic Lineages of Pathogenic Piroplasmids.Vet Sci. 2018 Apr 17;5(2):41. doi: 10.3390/vetsci5020041. Vet Sci. 2018. PMID: 29673170 Free PMC article.
-
First molecular detection of piroplasmids in non-hematophagous bats from Brazil, with evidence of putative novel species.Parasitol Res. 2021 Jan;120(1):301-310. doi: 10.1007/s00436-020-06985-w. Epub 2020 Nov 26. Parasitol Res. 2021. PMID: 33244622
-
Comparative Bioinformatics Analysis of Transcription Factor Genes Indicates Conservation of Key Regulatory Domains among Babesia bovis, Babesia microti, and Theileria equi.PLoS Negl Trop Dis. 2016 Nov 10;10(11):e0004983. doi: 10.1371/journal.pntd.0004983. eCollection 2016 Nov. PLoS Negl Trop Dis. 2016. PMID: 27832060 Free PMC article. Review.
Cited by
-
Genomic reconstruction and features of glycosylation pathways in the apicomplexan Cryptosporidium parasites.Front Mol Biosci. 2022 Nov 17;9:1051072. doi: 10.3389/fmolb.2022.1051072. eCollection 2022. Front Mol Biosci. 2022. PMID: 36465557 Free PMC article. Review.
-
Assessment of Babesia bovis 6cys A and 6cys B as components of transmission blocking vaccines for babesiosis.Parasit Vectors. 2021 Apr 20;14(1):210. doi: 10.1186/s13071-021-04712-7. Parasit Vectors. 2021. PMID: 33879245 Free PMC article.
-
In Silico Survey and Characterization of Babesia microti Functional and Non-Functional Proteases.Pathogens. 2021 Nov 10;10(11):1457. doi: 10.3390/pathogens10111457. Pathogens. 2021. PMID: 34832610 Free PMC article.
References
-
- Samuelson J., Banerjee S., Magnelli P., Cui J., Kelleher D.J., Gilmore R., Robbins P.W. The diversity of dolichol-linked precursors to Asn-linked glycans likely results from secondary loss of sets of glycosyltransferases. Proc. Natl. Acad. Sci. USA. 2005;102:1548–1553. doi: 10.1073/pnas.0409460102. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

