Cellular and Molecular Mechanisms of R/S-Roscovitine and CDKs Related Inhibition under Both Focal and Global Cerebral Ischemia: A Focus on Neurovascular Unit and Immune Cells
- PMID: 33429982
- PMCID: PMC7827530
- DOI: 10.3390/cells10010104
Cellular and Molecular Mechanisms of R/S-Roscovitine and CDKs Related Inhibition under Both Focal and Global Cerebral Ischemia: A Focus on Neurovascular Unit and Immune Cells
Abstract
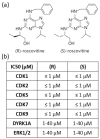
Ischemic stroke is the second leading cause of death worldwide. Following ischemic stroke, Neurovascular Unit (NVU) inflammation and peripheral leucocytes infiltration are major contributors to the extension of brain lesions. For a long time restricted to neurons, the 10 past years have shown the emergence of an increasing number of studies focusing on the role of Cyclin-Dependent Kinases (CDKs) on the other cells of NVU, as well as on the leucocytes. The most widely used CDKs inhibitor, (R)-roscovitine, and its (S) isomer both decreased brain lesions in models of global and focal cerebral ischemia. We previously showed that (S)-roscovitine acted, at least, by modulating NVU response to ischemia. Interestingly, roscovitine was shown to decrease leucocytes-mediated inflammation in several inflammatory models. Specific inhibition of roscovitine majors target CDK 1, 2, 5, 7, and 9 showed that these CDKs played key roles in inflammatory processes of NVU cells and leucocytes after brain lesions, including ischemic stroke. The data summarized here support the investigation of roscovitine as a potential therapeutic agent for the treatment of ischemic stroke, and provide an overview of CDK 1, 2, 5, 7, and 9 functions in brain cells and leucocytes during cerebral ischemia.
Keywords: CDK; ischemic stroke; leucocytes; neurovascular unit; roscovitine.
Conflict of interest statement
Serge Timsit is the co-inventor of a patent concerning (
Figures

References
-
- Johnson C.O., Nguyen M., Roth G.A., Nichols E., Alam T., Abate D., Abd-Allah F., Abdelalim A., Abraha H.N., Abu-Rmeileh N.M., et al. Global, Regional, and National Burden of Stroke, 1990–2016: A Systematic Analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:439–458. doi: 10.1016/S1474-4422(19)30034-1. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

