Tumor-Associated Antigen xCT and Mutant-p53 as Molecular Targets for New Combinatorial Antitumor Strategies
- PMID: 33430127
- PMCID: PMC7827209
- DOI: 10.3390/cells10010108
Tumor-Associated Antigen xCT and Mutant-p53 as Molecular Targets for New Combinatorial Antitumor Strategies
Abstract
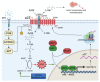
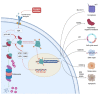
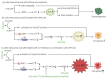
The cystine/glutamate antiporter xCT is a tumor-associated antigen that has been newly identified in many cancer types. By participating in glutathione biosynthesis, xCT protects cancer cells from oxidative stress conditions and ferroptosis, and contributes to metabolic reprogramming, thus promoting tumor progression and chemoresistance. Moreover, xCT is overexpressed in cancer stem cells. These features render xCT a promising target for cancer therapy, as has been widely reported in the literature and in our work on its immunotargeting. Interestingly, studies on the TP53 gene have revealed that both wild-type and mutant p53 induce the post-transcriptional down modulation of xCT, contributing to ferroptosis. Moreover, APR-246, a small molecule drug that can restore wild-type p53 function in cancer cells, has been described as an indirect modulator of xCT expression in tumors with mutant p53 accumulation, and is thus a promising drug to use in combination with xCT inhibition. This review summarizes the current knowledge of xCT and its regulation by p53, with a focus on the crosstalk of these two molecules in ferroptosis, and also considers some possible combinatorial strategies that can make use of APR-246 treatment in combination with anti-xCT immunotargeting.
Keywords: APR-246; PRIMA-1; breast cancer; p53; xCT.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Ruiu R., Rolih V., Bolli E., Barutello G., Riccardo F., Quaglino E., Merighi I.F., Pericle F., Donofrio G., Cavallo F., et al. Fighting breast cancer stem cells through the immune-targeting of the xCT cystine–glutamate antiporter. Cancer Immunol. Immunother. 2019;68:131–141. doi: 10.1007/s00262-018-2185-1. - DOI - PMC - PubMed
-
- Liu D.S., Duong C.P., Haupt S., Montgomery K.G., House C.M., Azar W.J., Pearson H.B., Fisher O.M., Read M., Guerra G.R., et al. Inhibiting the system xC-/glutathione axis selectively targets cancers with mutant-p53 accumulation. Nat. Commun. 2017;8:1–14. doi: 10.1038/ncomms14844. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

