Different Modulatory Effects of Four Methicillin-Resistant Staphylococcus aureus Clones on MG-63 Osteoblast-Like Cells
- PMID: 33430251
- PMCID: PMC7825699
- DOI: 10.3390/biom11010072
Different Modulatory Effects of Four Methicillin-Resistant Staphylococcus aureus Clones on MG-63 Osteoblast-Like Cells
Abstract
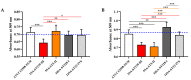
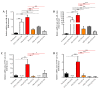
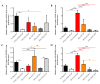
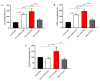
Staphylococcus aureus is a Gram-positive bacterium responsible for a variety of mild to life-threatening infections including bone infections such as osteomyelitis. This bacterium is able to invade and persist within non-professional phagocytic cells such as osteoblasts. In the present study, four different S. aureus strains, namely, 2SA-ST239-III (ST239), 5SA-ST5-II (ST5), 10SA-ST228-I (ST228), and 14SA-ST22-IVh (ST22), were tested for their ability to modulate cell viability in MG-63 osteoblast-like cells following successful invasion and persistence. Methicillin-sensitive S. aureus (MSSA) ATCC-12598-ST30 (ST30) was used as control strain. Despite being proven that ST30, ST239, and ST22 have a similar ability to internalize and persist in MG-63 osteoblast-like cells under our experimental conditions, we demonstrated that the observed decrease in cell viability was due to the different behavior of the considered strains, rather than the number of intracellular bacteria. We focused our attention on different biochemical cell functions related to inflammation, cell metabolism, and oxidative stress during osteoblast infections. We were able to show the following: (1) ST30 and ST239 were the only two clones able to persist and maintain their number in the hostile environment of the cell during the entire period of infection; (2) ST239 was the only clone able to significantly increase gene expression (3 and 24 h post-infection (p.i.)) and protein secretion (24 h p.i.) of both interleukin-6 (IL-6) and tumor necrosis factor alpha (TNF-α) in MG-63 osteoblast-like cells; (3) the same clone determined a significant up-regulation of the transforming growth factorbeta 1 (TGF-β1) and of the metabolic marker glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNAs at 24 h p.i.; and (4) neither the MSSA nor the four methicillin-resistant S. aureus (MRSA) strains induced oxidative stress phenomena in MG-63 cells, although a high degree of variability was observed for the different clones with regard to the expression pattern of nuclear factor E2-related factor 2 (Nrf2) and its downstream gene heme oxygenase 1 (HO-1) activation. Our results may pave the way for an approach to S. aureus-induced damage, moving towards individualized therapeutic strategies that take into account the differences between MSSA and MRSA as well as the distinctive features of the different clones. This approach is based on a change of paradigm in antibiotic therapy involving a case-based use of molecules able to counteract pro-inflammatory cytokines activity such as selective cytokine signaling inhibitors (IL-6, TNF-α).
Keywords: Staphylococcus aureus; cytokines; eukaryotic host–pathogen interaction; immune system; inflammation; internalization; osteoblast-like cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Staphylococcus aureus ST228 and ST239 as models for expression studies of diverse markers during osteoblast infection and persistence.Microbiologyopen. 2021 Mar;10(2):e1178. doi: 10.1002/mbo3.1178. Microbiologyopen. 2021. PMID: 33970534 Free PMC article.
-
The osteoblast secretome in Staphylococcus aureus osteomyelitis.Front Immunol. 2022 Nov 22;13:1048505. doi: 10.3389/fimmu.2022.1048505. eCollection 2022. Front Immunol. 2022. PMID: 36483565 Free PMC article. Review.
-
Detection of methicillin-resistant Staphylococcus aureus persistence in osteoblasts using imaging flow cytometry.Microbiologyopen. 2020 May;9(5):e1017. doi: 10.1002/mbo3.1017. Epub 2020 Apr 1. Microbiologyopen. 2020. PMID: 32237200 Free PMC article.
-
[Infectivity-resistotype-genotype clustering of methicillin-resistant Staphylococcus aureus strains in the Central Blacksea Region of Turkey].Mikrobiyol Bul. 2014 Jan;48(1):14-27. Mikrobiyol Bul. 2014. PMID: 24506712 Turkish.
-
Staphylococcus aureus vs. Osteoblast: Relationship and Consequences in Osteomyelitis.Front Cell Infect Microbiol. 2015 Nov 26;5:85. doi: 10.3389/fcimb.2015.00085. eCollection 2015. Front Cell Infect Microbiol. 2015. PMID: 26636047 Free PMC article. Review.
Cited by
-
Contemporary Clinical Isolates of Staphylococcus aureus from Pediatric Osteomyelitis Patients Display Unique Characteristics in a Mouse Model of Hematogenous Osteomyelitis.Infect Immun. 2021 Sep 16;89(10):e0018021. doi: 10.1128/IAI.00180-21. Epub 2021 Jun 7. Infect Immun. 2021. PMID: 34097469 Free PMC article.
-
Staphylococcus aureus ST228 and ST239 as models for expression studies of diverse markers during osteoblast infection and persistence.Microbiologyopen. 2021 Mar;10(2):e1178. doi: 10.1002/mbo3.1178. Microbiologyopen. 2021. PMID: 33970534 Free PMC article.
-
Generation and Characterization of Stable Small Colony Variants of USA300 Staphylococcus aureus in RAW 264.7 Murine Macrophages.Antibiotics (Basel). 2024 Mar 16;13(3):264. doi: 10.3390/antibiotics13030264. Antibiotics (Basel). 2024. PMID: 38534699 Free PMC article.
-
Immunomodulatory Effects of Chicken Broth and Histidine Dipeptides on the Cyclophosphamide-Induced Immunosuppression Mouse Model.Nutrients. 2022 Oct 25;14(21):4491. doi: 10.3390/nu14214491. Nutrients. 2022. PMID: 36364753 Free PMC article.
-
The osteoblast secretome in Staphylococcus aureus osteomyelitis.Front Immunol. 2022 Nov 22;13:1048505. doi: 10.3389/fimmu.2022.1048505. eCollection 2022. Front Immunol. 2022. PMID: 36483565 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
- RC: 2635256/Italian Ministry of Health Research Program 2018
- 2017SFBFER/Minister of research
- Identification of cancer driver genes for novel diagnostics and therapeutic strategies - Piano per la ricerca 2016-2018 - Linea di intervento 2/University of Catania, Dept. of Biomedical and Biotechnological Sciences
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

