Bromamine T (BAT) Exerts Stronger Anti-Cancer Properties than Taurine (Tau)
- PMID: 33430276
- PMCID: PMC7825693
- DOI: 10.3390/cancers13020182
Bromamine T (BAT) Exerts Stronger Anti-Cancer Properties than Taurine (Tau)
Abstract
Background: Taurine (Tau) ameliorates cancer pathogenesis. Researchers have focused on the functional properties of bromamine T (BAT), a stable active bromine molecule. Both N-bromotaurine (TauNHBr) and BAT exert potent anti-inflammatory properties, but the landscape remains obscure concerning the anti-cancer effect of BAT.
Methods: We used Crystal Violet, colony formation, flow cytometry and Western blot experiments to evaluate the effect of BAT and Tau on the apoptosis and autophagy of cancer cells. Xenograft experiments were used to determine the in vivo cytotoxicity of either agent.
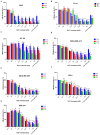
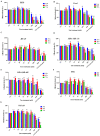
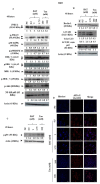
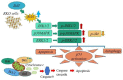
Results: We demonstrated that both BAT and Tau inhibited the growth of human colon, breast, cervical and skin cancer cell lines. Among them, BAT exerted the greatest cytotoxic effect on both RKO and MDA-MB-468 cells. In particular, BAT increased the phosphorylation of c-Jun N-terminal kinases (JNK½), p38 mitogen-activated protein kinase (MAPK), and extracellular-signal-regulated kinases (ERK½), thereby inducing mitochondrial apoptosis and autophagy in RKO cells. In contrast, Tau exerted its cytotoxic effect by upregulating JNK½ forms, thus triggering mitochondrial apoptosis in RKO cells. Accordingly, colon cancer growth was impaired in vivo.
Conclusions: BAT and Tau exerted their anti-tumor properties through the induction of (i) mitochondrial apoptosis, (ii) the MAPK family, and iii) autophagy, providing novel anti-cancer therapeutic modalities.
Keywords: breast cancer; bromamine T; colon cancer; taurine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








Similar articles
-
Bromamine T, a stable active bromine compound, prevents the LPS‑induced inflammatory response.Int J Mol Med. 2021 Apr;47(4):37. doi: 10.3892/ijmm.2021.4870. Epub 2021 Feb 4. Int J Mol Med. 2021. PMID: 33537817 Free PMC article.
-
Comparative Analysis of Microbicidal and Anti-inflammatory Properties of Novel Taurine Bromamine Derivatives and Bromamine T.Adv Exp Med Biol. 2017;975 Pt 1:515-534. doi: 10.1007/978-94-024-1079-2_41. Adv Exp Med Biol. 2017. PMID: 28849479
-
Activation of extracellular signal-regulated kinase and c-Jun-NH(2)-terminal kinase but not p38 mitogen-activated protein kinases is required for RRR-alpha-tocopheryl succinate-induced apoptosis of human breast cancer cells.Cancer Res. 2001 Sep 1;61(17):6569-76. Cancer Res. 2001. PMID: 11522656
-
Active, phosphorylation-dependent mitogen-activated protein kinase (MAPK/ERK), stress-activated protein kinase/c-Jun N-terminal kinase (SAPK/JNK), and p38 kinase expression in Parkinson's disease and Dementia with Lewy bodies.J Neural Transm (Vienna). 2001;108(12):1383-96. doi: 10.1007/s007020100015. J Neural Transm (Vienna). 2001. PMID: 11810403
-
Taurine induces the apoptosis of breast cancer cells by regulating apoptosis-related proteins of mitochondria.Int J Mol Med. 2015 Jan;35(1):218-26. doi: 10.3892/ijmm.2014.2002. Epub 2014 Nov 13. Int J Mol Med. 2015. PMID: 25395275
Cited by
-
FYCO1 Peptide Analogs: Design and Characterization of Autophagy Inhibitors as Co-Adjuvants in Taxane Chemotherapy of Prostate Cancer.Int J Mol Sci. 2025 Jun 3;26(11):5365. doi: 10.3390/ijms26115365. Int J Mol Sci. 2025. PMID: 40508175 Free PMC article.
-
Integrative analysis of taurine metabolism-related genes prognostic signature with immunotherapy and identification of ABCB1 and GORASP1 as key genes in nasopharyngeal carcinoma.Amino Acids. 2025 Apr 24;57(1):21. doi: 10.1007/s00726-025-03452-7. Amino Acids. 2025. PMID: 40272558 Free PMC article.
-
Bromamine T, a stable active bromine compound, prevents the LPS‑induced inflammatory response.Int J Mol Med. 2021 Apr;47(4):37. doi: 10.3892/ijmm.2021.4870. Epub 2021 Feb 4. Int J Mol Med. 2021. PMID: 33537817 Free PMC article.
References
-
- Di Nicolantonio F., Martini M., Molinari F., Sartore-Bianchi A., Arena S., Saletti P., De Dosso S., Mazzucchelli L., Frattini M., Siena S., et al. Wild-Type BRAF Is Required for Response to Panitumumab or Cetuximab in Metastatic Colorectal Cancer. J. Clin. Oncol. 2008;26:5705–5712. doi: 10.1200/JCO.2008.18.0786. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

