Lynch Syndrome: Its Impact on Urothelial Carcinoma
- PMID: 33430305
- PMCID: PMC7825811
- DOI: 10.3390/ijms22020531
Lynch Syndrome: Its Impact on Urothelial Carcinoma
Abstract
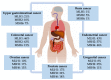
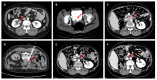
Lynch syndrome, known as hereditary nonpolyposis colorectal cancer (HNPCC), is an autosomal-dominant familial cancer syndrome with an increased risk for urothelial cancer (UC). Mismatch repair (MMR) deficiency, due to pathogenic variants in MLH1, MSH2, MSH6, and PMS2, and microsatellite instability, are known for development of Lynch syndrome (LS) associated carcinogenesis. UC is the third most common cancer type in LS-associated tumors. The diversity of germline variants in the affected MMR genes and their following subsequent function loss might be responsible for the variation in cancer risk, suggesting an increased risk of developing UC in MSH2 mutation carriers. In this review, we will focus on LS-associated UC of the upper urinary tract (UUT) and bladder, their germline profiles, and outcomes compared to sporadic UC, the impact of genetic testing, as well as urological follow-up strategies in LS. In addition, we present a case of metastatic LS-associated UC of the UUT and bladder, achieving complete response during checkpoint inhibition since more than 2 years.
Keywords: DNA mismatch repair genes; Lynch syndrome; MMR; checkpoint inhibitor; immunotherapy; microsatellite instability; upper urinary tract; urothelial cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Universal Lynch Syndrome Screening Should be Performed in All Upper Tract Urothelial Carcinomas.Am J Surg Pathol. 2018 Nov;42(11):1549-1555. doi: 10.1097/PAS.0000000000001141. Am J Surg Pathol. 2018. PMID: 30148743
-
Upper tract urothelial carcinomas: frequency of association with mismatch repair protein loss and lynch syndrome.Mod Pathol. 2017 Jan;30(1):146-156. doi: 10.1038/modpathol.2016.171. Epub 2016 Oct 7. Mod Pathol. 2017. PMID: 27713421
-
Clinicopathological characteristics of patients with upper urinary tract urothelial cancer with loss of immunohistochemical expression of the DNA mismatch repair proteins in universal screening.Int J Urol. 2018 Feb;25(2):151-156. doi: 10.1111/iju.13481. Epub 2017 Nov 22. Int J Urol. 2018. PMID: 29164703
-
The Clinical Outcomes Among Patients Under 60 Years Old with Lynch Syndrome: Variations Based on Different Mutation Patterns.Int J Mol Sci. 2025 Apr 4;26(7):3383. doi: 10.3390/ijms26073383. Int J Mol Sci. 2025. PMID: 40244260 Free PMC article. Review.
-
Update on Lynch syndrome genomics.Fam Cancer. 2016 Jul;15(3):385-93. doi: 10.1007/s10689-016-9882-8. Fam Cancer. 2016. PMID: 26873718 Free PMC article. Review.
Cited by
-
Pembrolizumab alters the tumor immune landscape in a patient with dMMR glioblastoma.medRxiv [Preprint]. 2023 Dec 26:2023.12.08.23299732. doi: 10.1101/2023.12.08.23299732. medRxiv. 2023. PMID: 38234786 Free PMC article. Preprint.
-
Morphological predictors for microsatellite instability in urothelial carcinoma.Diagn Pathol. 2021 Nov 20;16(1):106. doi: 10.1186/s13000-021-01168-2. Diagn Pathol. 2021. PMID: 34801034 Free PMC article.
-
Contemporary Issues in Urothelial Carcinoma of Upper Urinary Tract.Adv Anat Pathol. 2024 Mar 1;31(2):80-87. doi: 10.1097/PAP.0000000000000421. Epub 2023 Nov 27. Adv Anat Pathol. 2024. PMID: 38009077 Free PMC article.
-
Lynch Syndrome and MSI-H Cancers: From Mechanisms to "Off-The-Shelf" Cancer Vaccines.Front Immunol. 2021 Sep 24;12:757804. doi: 10.3389/fimmu.2021.757804. eCollection 2021. Front Immunol. 2021. PMID: 34630437 Free PMC article. Review.
-
The Current Progress and Future Options of Multiple Therapy and Potential Biomarkers for Muscle-Invasive Bladder Cancer.Biomedicines. 2023 Feb 13;11(2):539. doi: 10.3390/biomedicines11020539. Biomedicines. 2023. PMID: 36831075 Free PMC article. Review.
References
-
- Kloth M., Ruesseler V., Engel C., Koenig K., Peifer M., Mariotti E., Kuenstlinger H., Florin A., Rommerscheidt-Fuss U., Koitzsch U., et al. Activating ERBB2/HER2 mutations indicate susceptibility to pan-HER inhibitors in Lynch and Lynch-like colorectal cancer. Gut. 2016;65:1296–1305. doi: 10.1136/gutjnl-2014-309026. - DOI - PubMed
-
- Mangold E., Pagenstecher C., Friedl W., Mathiak M., Buettner R., Engel C., Loeffler M., Holinski-Feder E., Müller-Koch Y., Keller G., et al. Spectrum and frequencies of mutations in MSH2 and MLH1 identified in 1,721 German families suspected of hereditary nonpolyposis colorectal cancer. Int. J. Cancer. 2005;116:692–702. doi: 10.1002/ijc.20863. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

