Namodenoson in Advanced Hepatocellular Carcinoma and Child-Pugh B Cirrhosis: Randomized Placebo-Controlled Clinical Trial
- PMID: 33430312
- PMCID: PMC7825785
- DOI: 10.3390/cancers13020187
Namodenoson in Advanced Hepatocellular Carcinoma and Child-Pugh B Cirrhosis: Randomized Placebo-Controlled Clinical Trial
Abstract
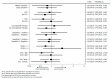
Namodenoson, an A3 adenosine-receptor agonist, showed promising results in advanced hepatocellular carcinoma (HCC) and moderate hepatic dysfunction (Child-Pugh B; CPB) in a phase I/II clinical study. This phase II study investigated namodenoson as second-line therapy in such patients. Patients were randomized 2:1 to twice a day (BID) namodenoson (25 mg; n = 50) or placebo (n = 28). The primary endpoint (overall survival [OS]) was not met. Median OS was 4.1/4.3 months for namodenoson/placebo (hazard ratio [HR], 0.82; 95% confidence interval [CI] 0.49-1.38; p = 0.46). Pre-planned subgroup analysis of CPB7 patients (34 namodenoson-treated, 22 placebo-treated) showed a nonsignificant improvement in OS/progression-free survival (PFS). OS: 6.9 versus 4.3 months; HR, 0.81; 95% CI: 0.45-1.43, p = 0.46. PFS: 3.5 versus 1.9 months; HR, 0.89; 95% CI: 0.51-1.55, p = 0.67 (log-rank test). The difference in 12-month OS was significant (44% versus 18%, p = 0.028). Response rates were determined in patients for whom ≥ 1 assessment post-baseline was available (34 namodenoson-treated, 21 placebo-treated). Partial response was achieved by 3/34 (8.8%) and 0/21 (0%) patients, respectively. Namodenoson was well-tolerated, with a safety profile comparable to that of the placebo group. No treatment-related deaths were reported; no patients withdrew due to toxicity. In conclusion, namodenoson demonstrated a favorable safety profile and a preliminary efficacy signal in HCC CPB.
Keywords: Child–Pugh B; hepatocellular carcinoma; namodenoson; overall survival; randomized clinical trial.
Conflict of interest statement
Salomon M. Stemmer and Michael H. Silverman are consultants and stakeholders at Can-Fite BioPharma; Zivit Harpaz, Motti Farbstein, Inbal Itzhak and Pnina Fishman are employed by and stakeholders at Can-Fite BioPharma; David Bristol is a consultant at Can-Fite BioPharma; The remaining authors declare no conflicts of interest.
Figures


References
-
- Global Burden of Disease Cancer Collaboration. Fitzmaurice C., Akinyemiju T.F., Al Lami F.H., Alam T., Alizadeh-Navaei R., Allen C., Alsharif U., Alvis-Guzman N., Amini E., et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2016: A systematic analysis for the global burden of disease study. JAMA Oncol. 2018;4:1553–1568. - PMC - PubMed
-
- FDA Website Sorafenib Package Insert. [(accessed on 4 January 2020)]; Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/021923s020lbl....
-
- NIH U.S. National Library of Medicine, Clinical Trials Data Base. [(accessed on 12 July 2020)]; Available online: ClinicalTrials.gov.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

