Heterofullerene MC59 (M = B, Si, Al) as Potential Carriers for Hydroxyurea Drug Delivery
- PMID: 33430313
- PMCID: PMC7825758
- DOI: 10.3390/nano11010115
Heterofullerene MC59 (M = B, Si, Al) as Potential Carriers for Hydroxyurea Drug Delivery
Abstract
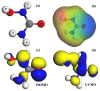
As a representative nanomaterial, C60 and its derivatives have drawn much attention in the field of drug delivery over the past years, due to their unique geometric and electronic structures. Herein, the interactions of hydroxyurea (HU) drug with the pristine C60 and heterofullerene MC59 (M = B, Si, Al) were investigated using the density functional theory calculations. The geometric and electronic properties in terms of adsorption configuration, adsorption energy, Hirshfeld charge, frontier molecular orbitals, and charge density difference are calculated. In contrast to pristine C60, it is found that HU molecule is chemisorbed on the BC59, SiC59, and AlC59 molecules with moderate adsorption energy and apparent charge transfer. Therefore, heterofullerene BC59, SiC59, and AlC59 are expected to be promising carriers for hydroxyurea drug delivery.
Keywords: DFT calculations; drug delivery; fullerene; hydroxyurea.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Yong Y.L., Lv S., Li X.H., Li T.W., Cui H.L. W@Si12 cluster as a potential sensor for CO and NO detection. Europhys. Lett. 2015;111:1. doi: 10.1209/0295-5075/111/10006. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

