Superparamagnetic Iron Oxide Particles (VSOPs) Show Genotoxic Effects but No Functional Impact on Human Adipose Tissue-Derived Stromal Cells (ASCs)
- PMID: 33430323
- PMCID: PMC7825809
- DOI: 10.3390/ma14020263
Superparamagnetic Iron Oxide Particles (VSOPs) Show Genotoxic Effects but No Functional Impact on Human Adipose Tissue-Derived Stromal Cells (ASCs)
Abstract
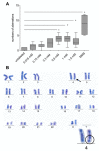
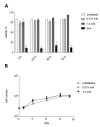
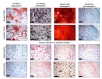
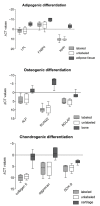
Adipose tissue-derived stromal cells (ASCs) represent a capable source for cell-based therapeutic approaches. For monitoring a cell-based application in vivo, magnetic resonance imaging (MRI) of cells labeled with iron oxide particles is a common method. It is the aim of the present study to analyze potential DNA damage, cytotoxicity and impairment of functional properties of human (h)ASCs after labeling with citrate-coated very small superparamagnetic iron oxide particles (VSOPs). Cytotoxic as well as genotoxic effects of the labeling procedure were measured in labeled and unlabeled hASCs using the MTT assay, comet assay and chromosomal aberration test. Trilineage differentiation was performed to evaluate an impairment of the differentiation potential due to the particles. Proliferation as well as migration capability were analyzed after the labeling procedure. Furthermore, the labeling of the hASCs was confirmed by Prussian blue staining, transmission electron microscopy (TEM) and high-resolution MRI. Below the concentration of 0.6 mM, which was used for the procedure, no evidence of genotoxic effects was found. At 0.6 mM, 1 mM as well as 1.5 mM, an increase in the number of chromosomal aberrations was determined. Cytotoxic effects were not observed at any concentration. Proliferation, migration capability and differentiation potential were also not affected by the procedure. Labeling with VSOPs is a useful labeling method for hASCs that does not affect their proliferation, migration and differentiation potential. Despite the absence of cytotoxicity, however, indications of genotoxic effects have been demonstrated.
Keywords: ASCs; MRI; VSOP; adipose tissue-derived stromal cells; cell labeling; iron oxide nanoparticles; toxicity.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures





Similar articles
-
Toxicity and Functional Impairment in Human Adipose Tissue-Derived Stromal Cells (hASCs) Following Long-Term Exposure to Very Small Iron Oxide Particles (VSOPs).Nanomaterials (Basel). 2020 Apr 13;10(4):741. doi: 10.3390/nano10040741. Nanomaterials (Basel). 2020. PMID: 32294970 Free PMC article.
-
DiI labeling of human adipose-derived stem cells: evaluation of DNA damage, toxicity and functional impairment.Cells Tissues Organs. 2013;197(5):384-98. doi: 10.1159/000346714. Epub 2013 Mar 9. Cells Tissues Organs. 2013. PMID: 23485626
-
Synthesis of acid-stabilized iron oxide nanoparticles and comparison for targeting atherosclerotic plaques: evaluation by MRI, quantitative MPS, and TEM alternative to ambiguous Prussian blue iron staining.Nanomedicine. 2015 Jul;11(5):1085-95. doi: 10.1016/j.nano.2015.01.002. Epub 2015 Feb 4. Nanomedicine. 2015. PMID: 25659644
-
Magnetic resonance imaging of ultrasmall superparamagnetic iron oxide-labeled exosomes from stem cells: a new method to obtain labeled exosomes.Int J Nanomedicine. 2016 Jun 1;11:2481-90. doi: 10.2147/IJN.S104152. eCollection 2016. Int J Nanomedicine. 2016. PMID: 27330291 Free PMC article.
-
Behaviour of adipose-derived canine mesenchymal stem cells after superparamagnetic iron oxide nanoparticles labelling for magnetic resonance imaging.BMC Vet Res. 2017 Feb 24;13(1):62. doi: 10.1186/s12917-017-0980-0. BMC Vet Res. 2017. PMID: 28235414 Free PMC article.
Cited by
-
Influence of Physicochemical Properties of Iron Oxide Nanoparticles on Their Antibacterial Activity.ACS Omega. 2024 Jul 25;9(31):33303-33334. doi: 10.1021/acsomega.4c02822. eCollection 2024 Aug 6. ACS Omega. 2024. PMID: 39130596 Free PMC article. Review.
-
Magnetic Nanoparticles Used in Oncology.Materials (Basel). 2021 Oct 10;14(20):5948. doi: 10.3390/ma14205948. Materials (Basel). 2021. PMID: 34683540 Free PMC article. Review.
-
Differentiation Behaviour of Adipose-Derived Stromal Cells (ASCs) Seeded on Polyurethane-Fibrin Scaffolds In Vitro and In Vivo.Biomedicines. 2021 Aug 9;9(8):982. doi: 10.3390/biomedicines9080982. Biomedicines. 2021. PMID: 34440186 Free PMC article.
-
Evaluation of In Vitro Cytotoxic, Genotoxic, Apoptotic, and Cell Cycle Arrest Potential of Iron-Nickel Alloy Nanoparticles.Toxics. 2022 Aug 24;10(9):492. doi: 10.3390/toxics10090492. Toxics. 2022. PMID: 36136457 Free PMC article.
References
-
- Froelich K., Hagen R., Kleinsasser N. Adipose-derived stromal cells (ASC)-basics and therapeutic approaches in otorhinolaryngology. Laryngorhinootologie. 2014;93:369–380. - PubMed
-
- Ren S., Chen J., Duscher D., Liu Y., Guo G., Kang Y., Xiong H., Zhan P., Wang Y., Wang C., et al. Microvesicles from human adipose stem cells promote wound healing by optimizing cellular functions via AKT and ERK signaling pathways. Stem Cel Res. Ther. 2019;10:47. doi: 10.1186/s13287-019-1152-x. - DOI - PMC - PubMed
-
- Matsumoto D., Sato K., Gonda K., Takaki Y., Shigeura T., Sato T., Aiba-Kojima E., Lizuka F., Inoue K., Suga H., et al. Cell-assisted lipotransfer: Supportive use of human adipose-derived cells for soft tissue augmentation with lipoinjection. Tissue Eng. 2006;12:3375–3382. doi: 10.1089/ten.2006.12.3375. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

