Combination of Cisplatin and Irradiation Induces Immunogenic Cell Death and Potentiates Postirradiation Anti-PD-1 Treatment Efficacy in Urothelial Carcinoma
- PMID: 33430352
- PMCID: PMC7825793
- DOI: 10.3390/ijms22020535
Combination of Cisplatin and Irradiation Induces Immunogenic Cell Death and Potentiates Postirradiation Anti-PD-1 Treatment Efficacy in Urothelial Carcinoma
Abstract
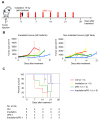
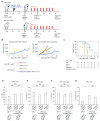
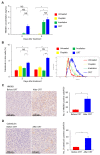
The therapeutic benefit of immune checkpoint inhibitor monotherapy is limited to a subset of patients in urothelial carcinoma (UC). Previous studies showed the immunogenicity of cisplatin and irradiation. Here, we investigated whether chemoradiotherapy (CRT), a combination of cisplatin and irradiation, could improve the efficacy of postirradiation anti-programmed cell death 1 (PD-1) treatment in UC. In our advanced UC patient cohort, patients with CRT showed a significantly better objective response rate (75%/22%) and overall survival (88%/30% at 12 months) following later pembrolizumab therapy compared to those without. Then, we created syngeneic UC mouse models by inoculating MB49 cells s.c. in C57BL/6J mice to examine the potential of CRT to enhance antitumor immunity in conjunction with postirradiation anti-PD-1 treatment. Nonirradiated tumors of the mice treated with CRT/postirradiation anti-PD-1 treatment had a significantly slower growth rate and a significantly higher expression of cytotoxic T cells compared to those of the mice treated with anti-PD-1 treatment alone. The mice treated with CRT/postirradiation anti-PD-1 treatment showed the best survival. Mechanistically, CRT provoked strong direct cytotoxicity and increased expressions of immunogenic cell death markers in MB49 cells. Therefore, the combination of cisplatin and irradiation induces immunogenic cell death and potentiates postirradiation anti-PD-1 treatment efficacy in UC.
Keywords: carcinoma; chemoradiotherapy; cisplatin; immunogenic cell death; immunotherapy; transitional cell.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

Similar articles
-
Tumor-treating fields (TTFields) induce immunogenic cell death resulting in enhanced antitumor efficacy when combined with anti-PD-1 therapy.Cancer Immunol Immunother. 2020 Jul;69(7):1191-1204. doi: 10.1007/s00262-020-02534-7. Epub 2020 Mar 6. Cancer Immunol Immunother. 2020. PMID: 32144446 Free PMC article.
-
Cisplatin Facilitates Radiation-Induced Abscopal Effects in Conjunction with PD-1 Checkpoint Blockade Through CXCR3/CXCL10-Mediated T-cell Recruitment.Clin Cancer Res. 2019 Dec 1;25(23):7243-7255. doi: 10.1158/1078-0432.CCR-19-1344. Epub 2019 Sep 10. Clin Cancer Res. 2019. PMID: 31506388
-
Neoadjuvant PD-L1 plus CTLA-4 blockade in patients with cisplatin-ineligible operable high-risk urothelial carcinoma.Nat Med. 2020 Dec;26(12):1845-1851. doi: 10.1038/s41591-020-1086-y. Epub 2020 Oct 12. Nat Med. 2020. PMID: 33046869 Free PMC article. Clinical Trial.
-
[First-line treatment of metastatic urothelial carcinoma : Update immuno-oncology].Urologe A. 2020 Jul;59(7):797-803. doi: 10.1007/s00120-020-01235-4. Urologe A. 2020. PMID: 32500171 Review. German.
-
Immunotherapy for Urothelial Carcinoma: Current Evidence and Future Directions.Curr Urol Rep. 2018 Nov 7;19(12):109. doi: 10.1007/s11934-018-0851-7. Curr Urol Rep. 2018. PMID: 30406502 Review.
Cited by
-
Immunogenic Cell Death Role in Urothelial Cancer Therapy.Curr Oncol. 2022 Sep 18;29(9):6700-6713. doi: 10.3390/curroncol29090526. Curr Oncol. 2022. PMID: 36135095 Free PMC article. Review.
-
Cisplatin-Based Combination Therapy for Enhanced Cancer Treatment.Curr Drug Targets. 2024;25(7):473-491. doi: 10.2174/0113894501294182240401060343. Curr Drug Targets. 2024. PMID: 38591210 Review.
-
Investigating cellular similarities and differences between upper tract urothelial carcinoma and bladder urothelial carcinoma using single-cell sequencing.Front Immunol. 2024 Jun 6;15:1298087. doi: 10.3389/fimmu.2024.1298087. eCollection 2024. Front Immunol. 2024. PMID: 38903524 Free PMC article.
-
Research progress in inducing immunogenic cell death of tumor cells.Front Immunol. 2022 Nov 17;13:1017400. doi: 10.3389/fimmu.2022.1017400. eCollection 2022. Front Immunol. 2022. PMID: 36466838 Free PMC article. Review.
-
Spatial Distribution and Predictive Significance of Dendritic Cells and Macrophages in Esophageal Cancer Treated With Combined Chemoradiotherapy and PD-1 Blockade.Front Immunol. 2022 Jan 3;12:786429. doi: 10.3389/fimmu.2021.786429. eCollection 2021. Front Immunol. 2022. PMID: 35046943 Free PMC article. Clinical Trial.
References
-
- Bellmunt J., von der Maase H., Mead G.M., Skoneczna I., De Santis M., Daugaard G., Boehle A., Chevreau C., Paz-Ares L., Laufman L.R., et al. Randomized phase III study comparing paclitaxel/cisplatin/gemcitabine and gemcitabine/cisplatin in patients with locally advanced or metastatic urothelial cancer without prior systemic therapy: EORTC Intergroup Study 30987. J. Clin. Oncol. 2012;30:1107–1113. doi: 10.1200/JCO.2011.38.6979. - DOI - PMC - PubMed
-
- Galsky M.D., Arija J.Á.A., Bamias A., Davis I.D., De Santis M., Kikuchi E., Garcia-Del-Muro X., De Giorgi U., Mencinger M., Izumi K., et al. Atezolizumab with or without chemotherapy in metastatic urothelial cancer (IMvigor130): A multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2020;395:1547–1557. doi: 10.1016/S0140-6736(20)30230-0. - DOI - PubMed
-
- Tamura D., Jinnouchi N., Abe M., Ikarashi D., Matsuura T., Kato R., Maekawa S., Kato Y., Kanehira M., Takata R., et al. Prognostic outcomes and safety in patients treated with pembrolizumab for advanced urothelial carcinoma: Experience in real-world clinical practice. Int. J. Clin. Oncol. 2020;25:899–905. doi: 10.1007/s10147-019-01613-9. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

