The Usefulness of Trivalent Phosphorus for the Synthesis of Dendrimers
- PMID: 33430439
- PMCID: PMC7827886
- DOI: 10.3390/molecules26020269
The Usefulness of Trivalent Phosphorus for the Synthesis of Dendrimers
Abstract
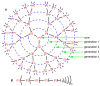
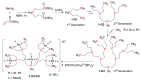
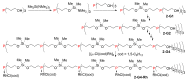
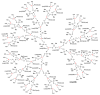
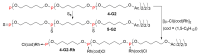
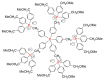
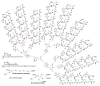
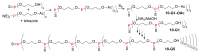
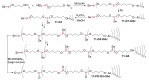
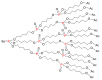
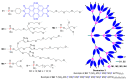
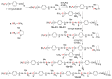
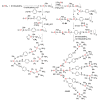
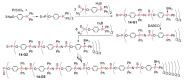
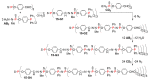
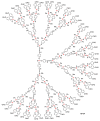
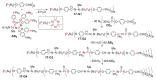
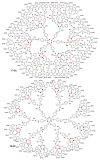
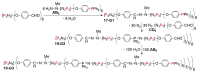
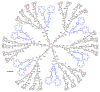
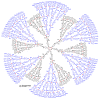
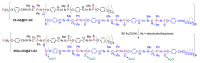
Dendrimers are hyperbranched macromolecules, which are synthesized step-by-step by the repetition of a series of reactions. While many different types of dendrimers are known, this review focusses on the use of trivalent phosphorus derivatives (essentially phosphines and phosphoramidites) for the synthesis of dendrimers. The first part presents dendrimers constituted of phosphines at each branching point. The other parts display the use of trivalent phosphorus derivatives during the synthesis of dendrimers. Different types of reactions have been applied to phosphines. The very first examples of phosphorus-containing dendrimers were obtained by the alkylation of phosphines. Then, several families of dendrimers were elaborated by reaction of phosphoramidites. Such a type of reaction is the base of the solid phase synthesis of oligonucleotides; it has been applied in particular for the synthesis of dendrimers constituted of oligonucleotides. Finally, the Staudinger reaction between phosphines and azides afforded different families of dendrimers, and was at the origin of accelerated methods of synthesis of dendrimers. Besides, the reactivity of the P=N-P=S linkages created by this reaction led to very original dendritic structures.
Keywords: Staudinger reaction; alkylation; dendrimers; phosphines; phosphoramidites.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



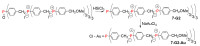



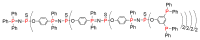









References
-
- Rengan K., Engel R. Phosphonium cascade molecules. J. Chem. Soc. Chem. Commun. 1990:1084–1085. doi: 10.1039/c39900001084. - DOI
-
- Caminade A.M., Laurent R., Turrin C.O., Rebout C., Delavaux-Nicot B., Ouali A., Zablocka M., Majoral J.P. Phosphorus dendrimers as viewed by P-31 NMR spectroscopy; synthesis and characterization. Comptes Rendus Chim. 2010;13:1006–1027. doi: 10.1016/j.crci.2010.03.008. - DOI
-
- Majoral J.-P., Caminade A.-M. Phosphorhydrazones as Useful Building Blocks for Special Architectures: Macrocycles and Dendrimers. Eur. J. Inorg. Chem. 2019:1457–1475. doi: 10.1002/ejic.201801184. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

