Polymorphisms in Pepsinogen C and miRNA Genes Associate with High Serum Pepsinogen II in Gastric Cancer Patients
- PMID: 33430456
- PMCID: PMC7827830
- DOI: 10.3390/microorganisms9010126
Polymorphisms in Pepsinogen C and miRNA Genes Associate with High Serum Pepsinogen II in Gastric Cancer Patients
Abstract
Background: Pepsinogen (PG) II (PGII) is a serological marker used to estimate the risk of gastric cancer but how PGII expression is regulated is largely unknown. It has been suggested that PGII expression, from the PGC (Progastricsin) gene, is regulated by microRNAs (miRNA), but how PGII levels vary with Helicobacter pylori (H. pylori) infection and miRNAs genotype remains unclear.
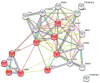
Methods: Serum levels of PGI and PGII were determined in 80 patients with gastric cancer and persons at risk for gastric cancer (74 first-degree relatives of patients, 62 patients with autoimmune chronic atrophic gastritis, and 2 patients with dysplasia), with and without H. pylori infection. As control from the general population, 52 blood donors were added to the analyses. Associations between PGII levels and genetic variants in PGC and miRNA genes in these groups were explored based on H. pylori seropositivity and the risk for gastric cancer. The two-dimensional difference in gel electrophoresis (2D-DIGE) and the NanoString analysis of messenger RNA (mRNAs) from gastric cancer tissue were used to determine the pathways associated with increased PGII levels.
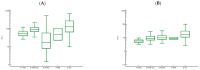
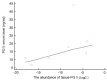
Results: PGII levels were significantly higher in patients with gastric cancer, and in those with H. pylori infection, than in other patients or controls. A PGI/PGII ratio ≤ 3 was found better than PGI < 25 ng/mL to identify patients with gastric cancer (15.0% vs. 8.8%). For two genetic variants, namely rs8111742 in miR-Let-7e and rs121224 in miR-365b, there were significant differences in PGII levels between genotype groups among patients with gastric cancer (p = 0.02 and p = 0.01, respectively), but not among other study subjects. Moreover, a strict relation between rs9471643 C-allele with H. pylori infection and gastric cancer was underlined. Fold change in gene expression of mRNA isolated from gastric cancer tissue correlated well with polymorphism, H. pylori infection, increased PGII level, and pathway for bacteria cell entry into the host.
Conclusions: Serum PGII levels depend in part on an interaction between H. pylori and host miRNA genotypes, which may interfere with the cut-off of PGI/PGII ratio used to identify persons at risk of gastric cancer. Results reported new findings regarding the relation among H. pylori, PGII-related host polymorphism, and genes involved in this interaction in the gastric cancer setting.
Keywords: Helicobacter pylori; PGC; RNAs; gastric cancer; polymorphisms; serum pepsinogen II.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
Figures




Similar articles
-
SNP-SNP interactions of three new pri-miRNAs with the target gene PGC and multidimensional analysis of H. pylori in the gastric cancer/atrophic gastritis risk in a Chinese population.Oncotarget. 2016 Apr 26;7(17):23700-14. doi: 10.18632/oncotarget.8057. Oncotarget. 2016. PMID: 26988755 Free PMC article.
-
Associations of Serum Pepsinogens and Helicobacter Pylori Infection with High-Sensitivity C-Reactive Protein in Medical Examination Population.Lab Med. 2021 Jan 4;52(1):57-63. doi: 10.1093/labmed/lmaa042. Lab Med. 2021. PMID: 32702129
-
The correlation between histological gastritis staging- 'OLGA/OLGIM' and serum pepsinogen test in assessment of gastric atrophy/intestinal metaplasia in China.Scand J Gastroenterol. 2017 Aug;52(8):822-827. doi: 10.1080/00365521.2017.1315739. Epub 2017 Apr 22. Scand J Gastroenterol. 2017. PMID: 28436254
-
Pepsinogen II in gastritis and Helicobacter pylori infection.Helicobacter. 2022 Apr;27(2):e12872. doi: 10.1111/hel.12872. Epub 2022 Jan 8. Helicobacter. 2022. PMID: 34997989 Review.
-
PGII Higher than PGI: a Case Analysis and Literature Review.Clin Lab. 2025 May 1;71(5). doi: 10.7754/Clin.Lab.2024.241104. Clin Lab. 2025. PMID: 40387737 Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

