A Novel Cyclic Pentadepsipeptide, N-Methylsansalvamide, Suppresses Angiogenic Responses and Exhibits Antitumor Efficacy against Bladder Cancer
- PMID: 33430488
- PMCID: PMC7827157
- DOI: 10.3390/cancers13020191
A Novel Cyclic Pentadepsipeptide, N-Methylsansalvamide, Suppresses Angiogenic Responses and Exhibits Antitumor Efficacy against Bladder Cancer
Abstract
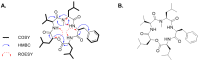
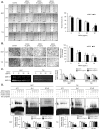
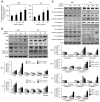
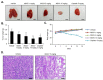
Here, we explored the anti-tumor efficacy of a cyclic pentadepsipeptide, N-methylsansalvamide (MSSV), in bladder cancer. MSSV inhibited the proliferation of both bladder cancer 5637 and T24 cells, which was attributed to the G1-phase cell cycle arrest, apoptosis induction, and alteration of mitogen-activated protein kinases (MAPKs) and protein kinase b (AKT) signaling pathways. Additionally, the treatment of bladder cancer cells with MSSV suppressed migratory and invasive potential via the transcription factor-mediated expression of matrix metalloproteinase 9 (MMP-9). MSSV abrogated vascular endothelial growth factor (VEGF)-induced angiogenic responses in vitro and in vivo. Furthermore, our result showed the potent anti-tumor efficacy of MSSV in a xenograft mouse model implanted with bladder cancer 5637 cells. Finally, acute toxicity test data obtained from blood biochemical test and liver staining indicated that the oral administration of MSSV at 2000 mg/kg caused no adverse cytotoxic effects. Our preclinical data described the potent anti-angiogenic and anti-tumor efficacy of MSSV and showed no signs of acute toxicity, thereby suggesting the putative potential of oral MSSV as a novel anti-tumor agent in bladder cancer treatment.
Keywords: MSSV; anti-angiogenesis; anti-tumor efficacy; bladder cancer; single oral dose of acute toxicity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Zachos I., Konstantinopoulos P.A., Tzortzis V., Gravas S., Karatzas A., Karamouzis M.V., Melekos M., Papavassiliou A.G. Systemic Therapy of Metastatic Bladder Cancer in the Molecular Era: Current Status and Future Promise. Expert Opin. Investig. Drugs. 2010;19:875–887. doi: 10.1517/13543784.2010.496450. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

