The transition to Xpert MTB/RIF ultra: diagnostic accuracy for pulmonary tuberculosis in Kampala, Uganda
- PMID: 33430790
- PMCID: PMC7802232
- DOI: 10.1186/s12879-020-05727-8
The transition to Xpert MTB/RIF ultra: diagnostic accuracy for pulmonary tuberculosis in Kampala, Uganda
Abstract
Background: The World Health Organization (WHO) has endorsed the next-generation Xpert MTB/RIF Ultra (Ultra) cartridge, and Uganda is currently transitioning from the older generation Xpert MTB/RIF (Xpert) cartridge to Ultra as the initial diagnostic test for pulmonary tuberculosis (TB). We assessed the diagnostic accuracy of Ultra for pulmonary TB among adults in Kampala, Uganda.
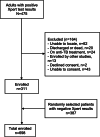
Methods: We sampled adults referred for Xpert testing at two hospitals and a health center over a 12-month period. We enrolled adults with positive Xpert and a random 1:1 sample with negative Xpert results. Expectorated sputum was collected for Ultra, and for solid and liquid culture testing for Xpert-negative patients. We measured sensitivity and specificity of Ultra overall and by HIV status, prior history of TB, and hospitalization, in reference to Xpert and culture results. We also assessed how classification of results in the new "trace" category affects Ultra accuracy.
Results: Among 698 participants included, 211 (30%) were HIV-positive and 336 (48%) had TB. The sensitivity of Ultra was 90.5% (95% CI 86.8-93.4) and specificity was 98.1% (95% CI 96.1-99.2). There were no significant differences in sensitivity and specificity by HIV status, prior history of TB or hospitalization. Xpert and Ultra results were concordant in 670 (96%) participants, with Ultra having a small reduction in specificity (difference 1.9, 95% CI 0.2 to 3.6, p=0.01). When "trace" results were considered positive for all patients, sensitivity increased by 2.1% (95% CI 0.3 to 3.9, p=0.01) without a significant reduction in specificity (- 0.8, 95% CI - 0.3 to 2.0, p=0.08).
Conclusions: After 1 year of implementation, Ultra had similar performance to Xpert. Considering "trace" results to be positive in all patients increased case detection without significant loss of specificity. Longitudinal studies are needed to compare the benefit of greater diagnoses to the cost of overtreatment.
Keywords: Diagnostics; Tuberculosis; Uganda; Xpert MTB/RIF; Xpert ultra.
Conflict of interest statement
The authors declare that they have no competing interests.
References
-
- Chakravorty S, Simmons AM, Rowneki M, Parmar H, Cao Y, Ryan J, et al. The new Xpert MTB/RIF ultra: improving detection of mycobacterium tuberculosis and resistance to rifampin in an assay suitable for point-of-care testing. MBio. 2017;8(4):e00812–e00817. doi: 10.1128/mBio.00812-17. - DOI - PMC - PubMed
-
- Dorman SE, Schumacher SG, Alland D, Nabeta P, Armstrong DT, King B, et al. Xpert MTB/RIF ultra for detection of mycobacterium tuberculosis and rifampicin resistance: a prospective multicentre diagnostic accuracy study. Lancet Infect Dis. 2018;18(1):76–84. doi: 10.1016/S1473-3099(17)30691-6. - DOI - PMC - PubMed
-
- World Health Organization. WHO meeting report of a technical expert consultation: Non-inferiority analysis of Xpert MTB/RIF Ultra compared to Xpert MTB/RIF. Available at: https://www.who.int/tb/publications/2017/XpertUltra/en/. Last accessed 4 Aug 2019.2017.
-
- World Health Organization. Global tuberculosis report 2019.2019 14 Dec 2019. Available from: https://apps.who.int/iris/bitstream/handle/10665/329368/9789241565714-en....
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources