Longitudinal analysis of cell-free mutated KRAS and CA 19-9 predicts survival following curative resection of pancreatic cancer
- PMID: 33430810
- PMCID: PMC7802224
- DOI: 10.1186/s12885-020-07736-x
Longitudinal analysis of cell-free mutated KRAS and CA 19-9 predicts survival following curative resection of pancreatic cancer
Abstract
Background: Novel biomarkers and molecular monitoring tools hold potential to improve outcome for patients following resection of pancreatic ductal adenocarcinoma (PDAC). We hypothesized that the combined longitudinal analysis of mutated cell-free plasma KRAS (cfKRASmut) and CA 19-9 during adjuvant treatment and follow-up might more accurately predict disease course than hitherto available parameters.
Methods: Between 07/2015 and 10/2018, we collected 134 plasma samples from 25 patients after R0/R1-resection of PDAC during adjuvant chemotherapy and post-treatment surveillance at our institution. Highly sensitive discriminatory multi-target ddPCR assays were employed to screen plasma samples for cfKRASmut. cfKRASmut and CA 19-9 dynamics were correlated with recurrence-free survival (RFS) and overall survival (OS). Patients were followed-up until 01/2020.
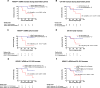
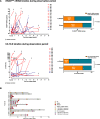
Results: Out of 25 enrolled patients, 76% had undergone R0 resection and 48% of resected PDACs were pN0. 17/25 (68%) of patients underwent adjuvant chemotherapy. Median follow-up was 22.0 months, with 19 out of 25 (76%) patients relapsing during study period. Median RFS was 10.0 months, median OS was 22.0 months. Out of clinicopathologic variables, only postoperative CA 19-9 levels and administration of adjuvant chemotherapy correlated with survival endpoints. cfKRASmut. was detected in 12/25 (48%) of patients, and detection of high levels inversely correlated with survival endpoint. Integration of cfKRASmut and CA 19-9 levels outperformed either individual marker. cfKRASmut outperformed CA 19-9 as dynamic marker since increase during adjuvant chemotherapy and follow-up was highly predictive of early relapse and poor OS.
Conclusions: Integrated analysis of cfKRASmut and CA 19-9 levels is a promising approach for molecular monitoring of patients following resection of PDAC. Larger prospective studies are needed to further develop this approach and dissect each marker's specific potential.
Keywords: Cell-free DNA (cfDNA); Circulating KRAS (cfKRAS mut); Droplet digital PCR (ddPCR); Liquid biopsy; Molecular monitoring; Pancreatic cancer; Prognostic biomarkers.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Bridgewater J, Lopes A, Wasan H, Malka D, Jensen L, Okusaka T, Knox J, Wagner D, Cunningham D, Shannon J, et al. Prognostic factors for progression-free and overall survival in advanced biliary tract cancer. Ann Oncol. 2016;27(1):134–140. - PubMed
-
- Huang L, Jansen L, Balavarca Y, Babaei M, van der Geest L, Lemmens V, Van Eycken L, De Schutter H, Johannesen TB, Primic-Žakelj M, et al. Stratified survival of resected and overall pancreatic cancer patients in Europe and the USA in the early twenty-first century: a large, international population-based study. BMC Med. 2018;16(1):125. - PMC - PubMed
-
- Wittel UA, Lubgan D, Ghadimi M, Belyaev O, Uhl W, Bechstein WO, Grützmann R, Hohenberger WM, Schmid A, Jacobasch L, et al. Consensus in determining the resectability of locally progressed pancreatic ductal adenocarcinoma – results of the Conko-007 multicenter trial. BMC Cancer. 2019;19(1):979. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

