Toward patient-centered tuberculosis preventive treatment: preferences for regimens and formulations in Lima, Peru
- PMID: 33430823
- PMCID: PMC7802335
- DOI: 10.1186/s12889-020-10098-5
Toward patient-centered tuberculosis preventive treatment: preferences for regimens and formulations in Lima, Peru
Abstract
Background: To ensure patient-centered tuberculosis preventive treatment, it is important to consider factors that make it easier for patients to complete treatment. However, there is little published literature about patient preferences for different preventive treatment regimen options, particularly from countries with high tuberculosis burdens.
Methods: We conducted a qualitative research study using a framework analysis approach to understand tuberculosis preventive treatment preferences among household contacts. We conducted three focus group discussions with 16 members of families affected by tuberculosis in Lima, Peru. Participants were asked to vote for preferred preventive treatment regimens and discuss the reasons behind their choices. Coding followed a deductive approach based on prior research, with data-driven codes added.
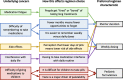
Results: In total, 7 (44%) participants voted for 3 months isoniazid and rifapentine, 4 (25%) chose 3 months isoniazid and rifampicin, 3 (19%) chose 4 months rifampicin, and 2 (13%) chose 6 months isoniazid. Preferences for shorter regimens over 6 months of isoniazid were driven by concerns over "getting tired" or "getting bored" of taking medications, the difficulty of remembering to take medications, side effects, and interference with daily life. For some, weekly dosing was perceived as being easier to remember and less disruptive, leading to a preference for 3 months isoniazid and rifapentine, which is dosed weekly. However, among caregivers, having a child-friendly formulation was more important than regimen duration. Caregivers reported difficulty in administering pills to children, and preferred treatments available as syrup or dispersible formulations.
Conclusions: There is demand for shorter regimens and child-friendly formulations for tuberculosis preventive treatment in high-burden settings. Individual preferences differ, suggesting that patient-centered care would best be supported by having multiple shorter regimens available.
Keywords: Chemoprophylaxis; Latent tuberculosis; Patient preference; Patient-centered care; TB preventive treatment.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Global Tuberculosis Report 2019. Geneva: World Health Organization; 2019.
-
- Cruz AT, Starke JR. Completion rate and safety of tuberculosis infection treatment with shorter regimens. Pediatrics. 2018;141(2):e20172838. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical