The leishmanicidal effect of Lucilia sericata larval saliva and hemolymph on in vitro Leishmania tropica
- PMID: 33430900
- PMCID: PMC7798311
- DOI: 10.1186/s13071-020-04543-y
The leishmanicidal effect of Lucilia sericata larval saliva and hemolymph on in vitro Leishmania tropica
Erratum in
-
Correction to: The leishmanicidal efect of Lucilia sericata larval saliva and hemolymph on in vitro Leishmania tropica.Parasit Vectors. 2021 Mar 15;14(1):155. doi: 10.1186/s13071-021-04649-x. Parasit Vectors. 2021. PMID: 33722266 Free PMC article. No abstract available.
Abstract
Background: Leishmaniasis is a major parasitic disease worldwide, except in Australia and Antarctica, and it poses a significant public health problem. Due to the absence of safe and effective vaccines and drugs, researchers have begun an extensive search for new drugs. The aim of the current study was to investigate the in vitro leishmanicidal activity of larval saliva and hemolymph of Lucilia sericata on Leishmania tropica.
Methods: The effects of different concentrations of larval products on promastigotes and intracellular amastigotes of L. tropica were investigated using the mouse cell line J774A.1 and peritoneal macrophages as host cells. The 3-(4.5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay and direct observation and counting method were used to assess the inhibitory effects and cell cytotoxicity of the larval products. The effects of larval products on the amastigote form of L. tropica were quantitatively estimated by calculating the rate of macrophage infection, number of amastigotes per infected macrophage cell, parasite load and survival index.
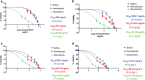
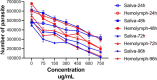
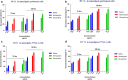
Results: The 50% cytotoxicity concentration (CC50) value of both larval saliva and hemolymph was 750 µg/ml, and the 50% inhibitory concentration (IC50) values were 134 µg/ml and 60 µg/ml for larval saliva and larval hemolymph, respectively. The IC50 for Glucantime, used a positive control, was (11.65 µg/ml). Statistically significant differences in viability percentages of promastigotes were observed for different doses of both larval saliva and hemolymph when compared with the negative control (p ≤ 0.0001). Microscopic evaluation of the amastigote forms revealed that treatment with 150 µg/ml larval hemolymph and 450 µg/ml larval saliva significantly decreased the rate of macrophage infection and the number of amastigotes per infected macrophage cell.
Conclusion: Larval saliva and hemolymph of L. sericata have acceptable leishmanicidal properties against L. tropica.
Keywords: Anti-leishmanial activity; Hemolymph; Intracellular amastigote; Leishmania tropica; Promastigote; Saliva.
Conflict of interest statement
The authors have stated explicitly that there are no conflicts of interest in connection with this article.
Figures


Similar articles
-
Evaluating leishmanicidal effects of Lucilia sericata products in combination with Apis mellifera honey using an in vitro model.PLoS One. 2023 Aug 3;18(8):e0283355. doi: 10.1371/journal.pone.0283355. eCollection 2023. PLoS One. 2023. PMID: 37535629 Free PMC article.
-
The therapeutic effect of larval saliva and hemolymph of Lucilia sericata on the treatment of Leishmania major lesion in BALB/c mice946.Parasit Vectors. 2023 Feb 16;16(1):72. doi: 10.1186/s13071-023-05660-0. Parasit Vectors. 2023. PMID: 36797798 Free PMC article.
-
Detection of anti-leishmanial effect of the Lucilia sericata larval secretions in vitro and in vivo on Leishmania tropica: first work.Exp Parasitol. 2012 Oct;132(2):129-34. doi: 10.1016/j.exppara.2012.06.004. Epub 2012 Jun 28. Exp Parasitol. 2012. PMID: 22750454
-
Evaluation of antileishmanial activity and cytotoxicity of the extracts of Berberis vulgaris and Nigella sativa against Leishmania tropica.J Vector Borne Dis. 2014 Dec;51(4):294-9. J Vector Borne Dis. 2014. PMID: 25540961
-
Formulation and Characterization of a Self-Emulsifying Drug Delivery System (SEDDS) of Curcumin for the Topical Application in Cutaneous and Mucocutaneous Leishmaniasis.Curr Top Med Chem. 2018;18(18):1603-1609. doi: 10.2174/1568026618666181025104818. Curr Top Med Chem. 2018. PMID: 30360717 Review.
Cited by
-
Correction to: The leishmanicidal efect of Lucilia sericata larval saliva and hemolymph on in vitro Leishmania tropica.Parasit Vectors. 2021 Mar 15;14(1):155. doi: 10.1186/s13071-021-04649-x. Parasit Vectors. 2021. PMID: 33722266 Free PMC article. No abstract available.
-
Therapeutic effects of Lucilia sericata larval excretion/secretion products on Leishmania major under in vitro and in vivo conditions.Parasit Vectors. 2022 Jun 16;15(1):212. doi: 10.1186/s13071-022-05322-7. Parasit Vectors. 2022. PMID: 35710519 Free PMC article.
-
Evaluating leishmanicidal effects of Lucilia sericata products in combination with Apis mellifera honey using an in vitro model.PLoS One. 2023 Aug 3;18(8):e0283355. doi: 10.1371/journal.pone.0283355. eCollection 2023. PLoS One. 2023. PMID: 37535629 Free PMC article.
-
The therapeutic effect of larval saliva and hemolymph of Lucilia sericata on the treatment of Leishmania major lesion in BALB/c mice946.Parasit Vectors. 2023 Feb 16;16(1):72. doi: 10.1186/s13071-023-05660-0. Parasit Vectors. 2023. PMID: 36797798 Free PMC article.
-
The in vitro and in vivo effects of Lucilia sericata larval secretions on Leishmania major.J Parasit Dis. 2023 Jun;47(2):363-368. doi: 10.1007/s12639-023-01574-x. Epub 2023 Apr 5. J Parasit Dis. 2023. PMID: 37193496 Free PMC article.
References
-
- Control of the leishmaniases: report of a meeting of the WHO Expert Commitee on the Control of Leishmaniases. Geneva: WHO technical report series, no: 949; 2010;1–186.
-
- de la Santé M. Leishmaniasis in high-burden countries: an epidemiological update based on data reported in 2014. Wkly Epidemiol Rec. 2016;91(22):286–296. - PubMed
-
- WHO. Leishmaniasis: key fact. 2020. Retrieved June 17, 2020 from: https://www.who.int/news-room/fact-sheets/detail/leishmaniasis.
-
- WHO: Department of control of neglected tropical diseases. Working to overcome the global impact of neglected tropical diseases: first WHO report on neglected tropical diseases. Geneva: World Health Organization, 2010 9241564091 Contract No.: WHO/HTM/NTD/2010.1.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

