A comparison of different community models of antiretroviral therapy delivery with the standard of care among stable HIV+ patients: rationale and design of a non-inferiority cluster randomized trial, nested in the HPTN 071 (PopART) study
- PMID: 33430928
- PMCID: PMC7802215
- DOI: 10.1186/s13063-020-05010-w
A comparison of different community models of antiretroviral therapy delivery with the standard of care among stable HIV+ patients: rationale and design of a non-inferiority cluster randomized trial, nested in the HPTN 071 (PopART) study
Abstract
Background: Following the World Health Organization's (WHO) 2015 guidelines recommending initiation of antiretroviral therapy (ART) irrespective of CD4 count for all people living with HIV (PLHIV), many countries in sub-Saharan Africa have adopted this strategy to reach epidemic control. As the number of PLHIV on ART rises, maintenance of viral suppression on ART for over 90% of PLHIV remains a challenge to government health systems in resource-limited high HIV burden settings. Non facility-based antiretroviral therapy (ART) delivery for stable HIV+ patients may increase sustainable ART coverage in resource-limited settings. Within the HPTN 071 (PopART) trial, two models, home-based delivery (HBD) or adherence clubs (AC), were offered to assess whether they achieved similar viral load suppression (VLS) to standard of care (SoC). In this paper, we describe the trial design and discuss the methodological issues and challenges.
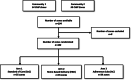
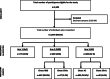
Methods: A three-arm cluster randomized non-inferiority trial, nested in two urban HPTN 071 trial communities in Zambia, randomly allocated 104 zones to SoC (35), HBD (35), or AC (34). ART and adherence support were delivered 3-monthly at home (HBD), adherence clubs (AC), or clinic (SoC). Adult HIV+ patients defined as "stable" on ART were eligible for inclusion. The primary endpoint was the proportion of PLHIV with virological suppression (≤ 1000 copies HIV RNA/ml) at 12 months (± 3months) after study entry across all three arms. Viral load measurement was done at the routine government laboratories in accordance with national guidelines, annually. The study was powered to determine if either of the community-based interventions would yield a viral suppression rate drop compared to SoC of no more than 5% in its absolute value. Both community-based interventions were delivered by community HIV providers (CHiPs). An additional qualitative study using observations, interviews with PLHIV, and FGDs with community HIV providers was nested in this study to complement the quantitative data.
Discussion: This trial was designed to provide rigorous randomized evidence of safety and efficacy of non-facility-based delivery of ART for stable PLHIV in high-burden resource-limited settings. This trial will inform policy regarding best practices and what is needed to strengthen scale-up of differentiated models of ART delivery in resource-limited settings.
Trial registration: ClinicalTrials.gov NCT03025165 . Registered on 19 January 2017.
Keywords: Adherence clubs; Anti-retroviral therapy; HIV; Home-based ART delivery; Zambia.
Conflict of interest statement
The authors declare they have no competing interests.
Figures
References
-
- World Health Organization. March 2014 supplement to the 2013 consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. Geneva: World Health Organization; 2014. - PubMed
-
- World Health Organization. Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. 2015. http://www.who.int/hiv/pub/guidelines/earlyrelease-arv/en/. Accessed 13 Mar 2017. - PubMed
-
- UNAIDS. Fact sheet - Latest statistics on the status of the AIDS epidemic. 2018. [Available from: http://www.unaids.org/en/resources/fact-sheet]. Accessed 4 Jan 2019.
-
- UNAIDS. Global AIDS Update. Geneva: UNAIDS; 2016. http://www.unaids.org/en/resources/documents/2016/Global-AIDS-update. Accessed 17 July 2018.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials