DTiGEMS+: drug-target interaction prediction using graph embedding, graph mining, and similarity-based techniques
- PMID: 33431036
- PMCID: PMC7325230
- DOI: 10.1186/s13321-020-00447-2
DTiGEMS+: drug-target interaction prediction using graph embedding, graph mining, and similarity-based techniques
Abstract
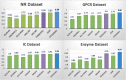
In silico prediction of drug-target interactions is a critical phase in the sustainable drug development process, especially when the research focus is to capitalize on the repositioning of existing drugs. However, developing such computational methods is not an easy task, but is much needed, as current methods that predict potential drug-target interactions suffer from high false-positive rates. Here we introduce DTiGEMS+, a computational method that predicts Drug-Target interactions using Graph Embedding, graph Mining, and Similarity-based techniques. DTiGEMS+ combines similarity-based as well as feature-based approaches, and models the identification of novel drug-target interactions as a link prediction problem in a heterogeneous network. DTiGEMS+ constructs the heterogeneous network by augmenting the known drug-target interactions graph with two other complementary graphs namely: drug-drug similarity, target-target similarity. DTiGEMS+ combines different computational techniques to provide the final drug target prediction, these techniques include graph embeddings, graph mining, and machine learning. DTiGEMS+ integrates multiple drug-drug similarities and target-target similarities into the final heterogeneous graph construction after applying a similarity selection procedure as well as a similarity fusion algorithm. Using four benchmark datasets, we show DTiGEMS+ substantially improves prediction performance compared to other state-of-the-art in silico methods developed to predict of drug-target interactions by achieving the highest average AUPR across all datasets (0.92), which reduces the error rate by 33.3% relative to the second-best performing model in the state-of-the-art methods comparison.
Keywords: Bioinformatics; Cheminformatics; Drug repositioning; Drug–target interaction; Graph embedding; Heterogenous network; Machine learning; Similarity integration; Similarity-based.
Conflict of interest statement
The authors have declared that no conflict of interests exist.
Figures




References
-
- DiMasi JA, Hansen RW, Grabowski HG. The price of innovation: new estimates of drug development costs. J Health Econ. 2003;22(2):151–185. - PubMed
-
- Yıldırım MA, et al. Drug–target network. Nat Biotechnol. 2007;25:1119. - PubMed
-
- Ashburn TT, Thor KB. Drug repositioning: identifying and developing new uses for existing drugs. Nat Rev Drug Discov. 2004;3(8):673–683. - PubMed
-
- Cheng AC, et al. Structure-based maximal affinity model predicts small-molecule druggability. Nat Biotechnol. 2007;25(1):71–75. - PubMed
-
- Alonso H, Bliznyuk AA, Gready JE. Combining docking and molecular dynamic simulations in drug design. Med Res Rev. 2006;26(5):531–568. - PubMed
Grants and funding
- BAS/1/1606-01-01/King Abdullah University of Science and Technology
- BAS/1/1059-01-01/King Abdullah University of Science and Technology
- BAS/1/1624-01-01/King Abdullah University of Science and Technology
- FCC/1/1976-26-01/King Abdullah University of Science and Technology
- FCC/1/1976-17-01/King Abdullah University of Science and Technology
LinkOut - more resources
Full Text Sources

