Bisphenol A promotes stress granule assembly and modulates the integrated stress response
- PMID: 33431410
- PMCID: PMC7823164
- DOI: 10.1242/bio.057539
Bisphenol A promotes stress granule assembly and modulates the integrated stress response
Abstract
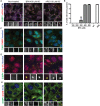
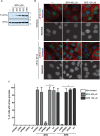
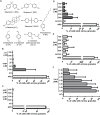
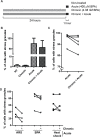
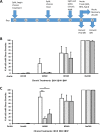
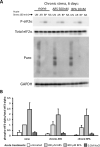
Bisphenol-A (BPA) is a ubiquitous precursor of polycarbonate plastics that is found in the blood and serum of >92% of Americans. While BPA has been well documented to act as a weak estrogen receptor (ER) agonist, its effects on cellular stress are unclear. Here, we demonstrate that high-dose BPA causes stress granules (SGs) in human cells. A common estrogen derivative, β-estradiol, does not trigger SGs, indicating the mechanism of SG induction is not via the ER pathway. We also tested other structurally related environmental contaminants including the common BPA substitutes BPS and BPF, the industrial chemical 4-nonylphenol (4-NP) and structurally related compounds 4-EP and 4-VP, as well as the pesticide 2,4-dichlorophenoxyacetic acid (2,4-D). The variable results from these related compounds suggest that structural homology is not a reliable predictor of the capacity of a compound to cause SGs. Also, we demonstrate that BPA acts primarily through the PERK pathway to generate canonical SGs. Finally, we show that chronic exposure to a low physiologically relevant dose of BPA suppresses SG assembly upon subsequent acute stress. Interestingly, this SG inhibition does not affect phosphorylation of eIF2α or translation inhibition, thus uncoupling the physical assembly of SGs from translational control. Our work identifies additional effects of BPA beyond endocrine disruption that may have consequences for human health.
Keywords: Bisphenol-A; Integrated stress response; Stress granules; Translational control.
© 2021. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures

References
-
- Apau J., Acheampong A. and Adua E (2018). Exposure to Bisphenol A, Bisphenol F, and Bisphenol S can result in obesity in human body. Cogent Chem. 4, 1-7. 10.1080/23312009.2018.1506601 - DOI
-
- Asimakopoulos A. G. and Thomaidis N. S. (2015). Bisphenol A, 4-t-octylphenol, and 4-nonylphenol determination in serum by hybrid solid phase extraction-precipitation technology technique tailored to liquid chromatography-tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 986-987, 85-93. 10.1016/j.jchromb.2015.02.009 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

