Noncanonical Auxin Signaling
- PMID: 33431583
- PMCID: PMC8091950
- DOI: 10.1101/cshperspect.a039917
Noncanonical Auxin Signaling
Abstract
Auxin influences all aspects of plant growth and development and exerts its function at scales ranging from the subcellular to the whole-organism level. A canonical mechanism for auxin signaling has been elucidated, which is based on derepression of downstream genes via ubiquitin-mediated degradation of transcriptional repressors. While the combinatorial nature of this canonical pathway provides great potential for specificity in the auxin response, alternative noncanonical signaling pathways required to mediate certain processes have been identified. One such pathway affects gene regulation in a manner that is reminiscent of mechanisms employed in animal hormone signaling, while another triggers transcriptional changes through auxin perception at the plasma membrane and the stabilization of transcriptional repressors. In some cases, the exact perception mechanisms and the nature of the receptors involved are yet to be revealed. In this review, we describe and discuss current knowledge on noncanonical auxin signaling and highlight unresolved questions surrounding auxin biology.
Copyright © 2021 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures




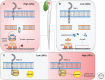
References
-
- Bergfeld R, Speth V, Schopfer P. 1988. Reorientation of microfibrils and microtubules at the outer epidermal wall of maize coleoptiles during auxin-mediated growth. Bot Acta 101: 57–67. 10.1111/j.1438-8677.1988.tb00012.x - DOI
-
- Berleth T, Jürgens G. 1993. The role of the monopteros gene in organizing the basal body region of the Arabidopsis embryo. Development 118: 575–587.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
